Circular Economy Assessment in Recycling of LLDPE Bags According to European Resolution, Thermal and Structural Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Samples Processing
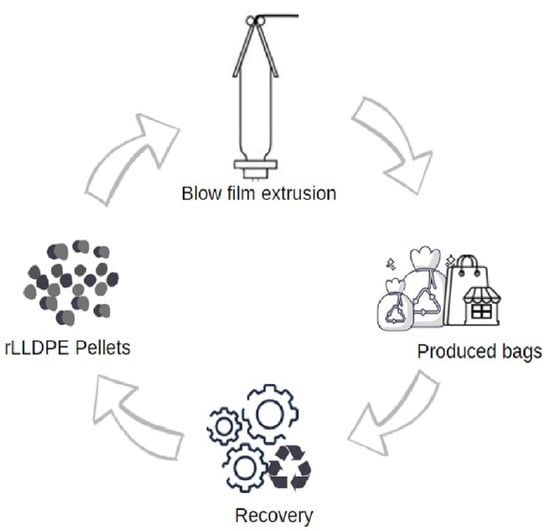
2.2. Extrusion and Blowing to Obtain the Film
2.3. Recycling to Obtain the Pellets
2.4. Calorimetric Analysis by DSC
2.5. Structural Characterization by FTIR
2.6. Tear Resistance Test
3. Results and Discussion
3.1. Calorimetric Analysis
3.1.1. Crystallization
3.1.2. Melting Temperature
3.1.3. Crystallinity
3.1.4. Oxidation Temperature
3.2. Structural Analysis FT-IR
3.3. Tear Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jubinville, D.; Esmizadeh, E.; Saikrishnan, S.; Tzoganakis, C.; Mekonnen, T. A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications. Sustain. Mater. Technol. 2020, 25, 188. [Google Scholar] [CrossRef]
- European Parliament and Council Directive 94/62/EC of 20 December 1994 on Packaging and Packaging Waste. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31994L0062 (accessed on 11 January 2022).
- Ellen Macarthur Foundation 2013 towards the Circular Economy. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/publications/Ellen-MacArthur-Foundation-Towards-the-Circular-Economy-vol.1.pdf (accessed on 11 January 2022).
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24. [Google Scholar] [CrossRef] [PubMed]
- Delva, L.; Van Kets, K.; Kuzmanovic, M.; Demets, R.; Hubo, S.; Mys, N.; De Meester, S.; Ragaert, K. Mechanical Recycling of Polymers for Dummies. Available online: http://ubuntoo-assets.s3.amazonaws.com/uploads/blog_document/document/8/Mechanical_Recycling_of_Polymers_for_Dummies.pdf (accessed on 11 January 2022).
- Colom, X.; Cañavate, X.J.; Suñol, J.J.; Pagès, P.; Saurina, J.; Carrasco, F. Natural and Artificial Aging of Polypropylene–Polyethylene Copolymers. J. Appl. Polym. Sci. 2003, 87, 1685. [Google Scholar] [CrossRef]
- Jin, H.; González-Gutierrez, J.; Oblak, P.; Zupančič, B.; Emri, I. The effect of extensive mechanical recycling on the properties of low density polyethylene. Polym. Degrad. Stab. 2012, 97, 2262. [Google Scholar] [CrossRef]
- Oblak, P.; Gonzalez-Gutierrez, J.; Zupančič, B.; Aulova, A.; Emri, I. Processability and mechanical properties of extensively recycled high density polyethylene. Polym. Degrad. Stab. 2015, 114, 133. [Google Scholar] [CrossRef]
- Cruz, S.A.; Zanín, M. Evaluation and identification of degradative processes in post-consumer recycled high-density polyethylene. Polym. Degrad. Stab. 2003, 80, 31. [Google Scholar] [CrossRef]
- Horodytska, O.; Valdés, F.J.; Fulana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413. [Google Scholar] [CrossRef]
- Dow Chemical. Dowlex 2645.01G Polyethylene Resin. Available online: https://www.dow.com/es-es/pdp.dowlex-264501g-polyethylene-resin.105779z.html (accessed on 11 January 2022).
- Ndiripo, A.; Eselem Bungu, P.S.; Pasch, H. Comprehensive branching analysis of polyethylene by combined fractionation and thermal analysis. Polym. Int. 2019, 68, 206. [Google Scholar] [CrossRef]
- Zerbi, G.; Gallino, G.; Del Fanti, N.; Baini, L. Structural depth profiling in polyethylene films by multiple internal reflection infra-red spectroscopy. Polymer 1989, 30, 2324. [Google Scholar] [CrossRef]
- Ballestar, R.; Pradas, C.; Puig Bosch, P.; Cañavate, F.J.; Colom, X. Physical-Thermal characterization of the degradation process during the manufacturing of an LLDPE film to produce bags. AFINIDAD 2021, 58, 177. [Google Scholar]
- Al-Salem, S.M.; Bumajdad, A.; Khan, A.R.; Sharma, B.K.; Chandrasekaran, S.R.; Al-Turki, F.A.; Al-Dhafeeri, A.T. Non-Isothermal degradation kinetics of virgin linear low density polyethylene (LLDPE) and biodegradable polymer blends. J. Polym. Res. 2018, 25, 1. [Google Scholar] [CrossRef]
- Craig, I.H.; White, J.R. Crystallization and chemi-crystallization of recycled photodegraded polyethylenes. Polym. Eng. Sci. 2005, 25, 588. [Google Scholar] [CrossRef]
- Zhang, X.M.; Elkoun, S.; Ajji, A.; Huneault, M.A. Oriented structure and anisotropy properties of polymer blown films: HDPE, LLDPE and LDPE. Polymer 2004, 45, 217. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Q.; Li, L.; Chen, W.; Wang, D.; Meng, L. Synergistic and competitive effects of temperature and flow on crystallization of polyethylene during film blowing. ACS Appl. Polym. Mater. 2019, 1, 1590. [Google Scholar] [CrossRef]
- Van Drofelen, M.; Roozemond, P.C.; Troisi, E.M.; Doufas, A.K.; Peters, G.W.M. Characterization of the primary and secondary crystallization kinetics of a lineal low-density polyethylene in quiescient- and flow-conditions. Polymer 2015, 76, 254. [Google Scholar] [CrossRef]
- Zhang, W.; Larson, R.G. Direct All-Atom Molecular Dynamics Simulations of the Effects of Short Chain Branching on Polyethylene Oligomer Crystal Nucleation. Macromolecules 2018, 51, 4762. [Google Scholar] [CrossRef]
- Saikrishnan, S.; Jubinville, D.; Tzoganakis, C.; Mekonnen, T.H. Thermo-Mechanical degradation of polypropylene (PP) and low-density polyethylene (LDPE) blends exposed to simulated recycling. Polym. Degrad. Stab. 2020, 182, 109390. [Google Scholar] [CrossRef]
- Hussein, I.A. Nonisothermal Crystallization Kinetics of Linear Metallocene Polyethylenes. J. Appl. Polym. Sci. 2008, 107, 20802. [Google Scholar]
- Pagés, P.; Carrasco, F.; Saurina, J.; Colom, X. FTIR and DSC Study of HDPE Structural Changes and Mechanical Properties Variation When Exposed to Weathering Aging During Canadian Winter. J. Appl. Polym. Sci. 1996, 60, 153. [Google Scholar] [CrossRef]
- Han, S.O.; Lee, D.W.; Han, O.H. Thermal degradation of crosslinked high density polyethylene. Polym. Degrad. Stab. 1999, 63, 237. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, B.; Ji, G.; Xu, Y. Studies on Nonisothermal Crystallization of Ultra-High Molecular Weight Polyethylene in Liquid Paraffin. J. Appl. Polym. Sci. 2006, 99, 2782. [Google Scholar] [CrossRef]
- Pathaweeisariyakul, T.; Nrkchamnan, K.; Thitisuk, B.; Yau, W. Methods of long chain branching detecition in PE by triple-detector gel permeation chromatography. J. Appl. Polym. Sci. 2015, 132, 128. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagés, P.; Saurina, J.; Colom, X. Estudio mediante FTIR y DSC de los cambios de cristalinidad del HDPE inducidos por condiciones climáticas drásticas. AFINIDAD 1995, 52, 231. [Google Scholar]
- Mendes, A.A.; Cunha, A.M.; Bernardo, C.A. Study of degradation mechanisms of polyethylene during reprocesssing. Polym. Degrad. Stab. 2011, 96, 1125. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Su, F.; Ji, Y.; Ali, S.; Zhao, H.; Meng, M.; Li, L. From molecular entanglement network to Crystal-Cross-Linked Network and Crystal Scaffold during film Blowing of polyethylene: An in Situ Synchorotron Radiation Small-and Wide-Angle X-Ray Scattering Study. Macromolecules 2018, 51, 4350. [Google Scholar] [CrossRef]
- Focke, W.W.; Van der Westhuizen, I. Oxidation induction time and oxidation onset temperature of polyethylene in air: Testing Gimzewski’s postulate. J. Therm. Anal. Calorim. 2010, 99, 285. [Google Scholar] [CrossRef]
- Schnabel, M. Polymer Degradation Principles and Practical Applications. J. Macromol. Sci. 1981, 19, 797. [Google Scholar]
- Cañavate, J.; Pages, P.; Saurina, J.; Colom, X.; Carrasco, F. Determination of small interactions in polymer composites by means of FTIR and DSC. Polym. Bull. 2000, 44, 293. [Google Scholar] [CrossRef]









| Sample | Processing Step | Tc (CS1) | Tc (CS2) | Tm (CS1) | Tm (CS2) |
|---|---|---|---|---|---|
| Pellets | 0 | - | 104.33 | 111.35 | 121.34 |
| 2 | - | 106.20 | 111.81 | 121.20 | |
| 4 | 101.62 | 106.67 | 113.41 | 122.05 | |
| 6 | 103.10 | 107.48 | 114.72 | 122.22 | |
| 8 | 101.73 | 108.34 | 115.34 | 120.75 | |
| 10 | 102.44 | 109.37 | 115.11 | 120.95 | |
| 12 | 102.52 | 109.35 | 116.44 | 121.33 | |
| Film | 1 | - | 104.89 | 111.14 | 121.76 |
| 3 | 101.05 | 107.05 | 113.70 | 120.34 | |
| 5 | 99.95 | 106.84 | 115.22 | 121.49 | |
| 7 | 100.14 | 106.60 | 116.32 | 120.32 | |
| 9 | 99.73 | 107.40 | 117.73 | 122.09 | |
| 11 | 100.44 | 108.38 | 118.10 | 121.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballestar, R.; Pradas, C.; Carrillo-Navarrete, F.; Cañavate, J.; Colom, X. Circular Economy Assessment in Recycling of LLDPE Bags According to European Resolution, Thermal and Structural Characterization. Polymers 2022, 14, 754. https://doi.org/10.3390/polym14040754
Ballestar R, Pradas C, Carrillo-Navarrete F, Cañavate J, Colom X. Circular Economy Assessment in Recycling of LLDPE Bags According to European Resolution, Thermal and Structural Characterization. Polymers. 2022; 14(4):754. https://doi.org/10.3390/polym14040754
Chicago/Turabian StyleBallestar, Ricardo, Celia Pradas, Fernando Carrillo-Navarrete, Javier Cañavate, and Xavier Colom. 2022. "Circular Economy Assessment in Recycling of LLDPE Bags According to European Resolution, Thermal and Structural Characterization" Polymers 14, no. 4: 754. https://doi.org/10.3390/polym14040754