Surface Modification of Sulfur-Assisted Reduced Graphene Oxide with Poly(phenylene sulfide) for Multifunctional Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SrGO
2.3. In Situ Polymerization of PPS/SrGO and PPS/GO Composites
2.4. Preparation of Diluted PPS Composites by MB
2.5. Classical Micromechanics for Effective Thermal Conductivity (TC)
2.6. Characterization
3. Results
3.1. Preparation of SrGO
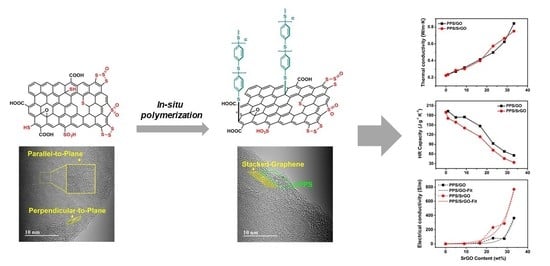
3.2. In-Situ Polymerization and Characterization of PPS/GO and PPS/SrGO Composites
3.3. Physical Properties of PPS/GO and PPS/SrGO Composites
3.4. Application of PPS/SrGO Composite as MB Using High Dispersibility of SrGO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peiyuan, Z.; Abbas, T.; Mohammadali, S.; Joseph, F.; Farid, B. Overall Investigation of Poly (Phenylene Sulfide) from Synthesis and Process to Applications—A Review. Macromol. Mater. Eng. 2019, 304, 1800686–1800712. [Google Scholar]
- Ashok, S.R.; Kailash, R.N.; Sandeep, A.W. Polyphenylene Sulfide (PPS): State of the Art and Applications. Rev. Chem. Eng. 2013, 29, 471–489. [Google Scholar]
- Roberto, N.; Beniamino, P.; Antonio, S. Crystal Structure of Poly (p-phenylene sulfide): A Refinement by X-ray Measurements and Molecular Mechanics Calculations. Macromolecules 1999, 32, 7682–7687. [Google Scholar]
- Silvestre, C.; Pace, E.D.; Napolitano, R.; Pirozzi, B.; Cesario, G. Crystallization, Morphology, and Thermal Behavior of Poly (p-phenylene sulfide). J. Polym. Sci. B Polym. Phys. 2001, 39, 415–424. [Google Scholar] [CrossRef]
- Luo, D.; Chen, M.; Xu, J.; Yin, X.; Wu, J.; Chen, S.; Wang, L. Polyphenylene sulfide nonwoven-based composite separator with superior heat-resistance and flame retardancy for high power lithium ion battery. Compos. Sci. Technol. 2018, 157, 119–125. [Google Scholar] [CrossRef]
- Maaroufi, M.A.; Carpier, Y.; Vieille, B.; Gilles, L.; Coppalle, A.; Barbe, F. Post-fire compressive behaviour of carbon fibers woven-ply Polyphenylene Sulfide laminates for aeronautical applications. Compos. B Eng. 2017, 119, 101–113. [Google Scholar] [CrossRef]
- Vieille, B.; Lefebvre, C.; Coppalle, A. Post fire behavior of carbon fibers Polyphenylene Sulfide- and epoxy-based laminates for aeronautical applications: A comparative study. Mater. Des. 2014, 63, 56–68. [Google Scholar]
- Vieille, B.; Coppalle, A.; Keller, C.; Garda, M.R.; Viel, Q.; Dargent, E. Correlation between post fire behavior and microstructure degradation of aeronautical polymer composites. Mater. Des. 2015, 74, 76–85. [Google Scholar]
- Junchen, L.; Jiaxiang, Q.; Yudi, M.; Shuanjin, W.; Dongmei, H.; Min, X.; Yuezhong, M. Polyphenylene Sulfide Separator for High Safety Lithium-Ion Batteries. J. Electrochem. Soc. 2019, 166, A1644–A1652. [Google Scholar]
- Yoo, T.J.; Hwang, E.-B.; Jeong, Y.G. Thermal and electrical properties of poly (phenylene sulfide)/carbon nanotube nanocomposite films with a segregated structure. Compos. A Appl. Sci. Manuf. 2016, 91, 77–84. [Google Scholar] [CrossRef]
- Pak, S.Y.; Kim, H.M.; Kim, S.Y.; Youn, J.R. Synergistic improvement of thermal conductivity of thermoplastic composites with mixed boron nitride and multi-walled carbon nanotube fillers. Carbon 2012, 50, 4830–4838. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Guan, J.; Simard, B.; Gómez-Fatou, M.A. Poly (phenylene sulphide) and poly (ether ether ketone) composites reinforced with single-walled carbon nanotube buckypaper: II–Mechanical properties, electrical and thermal conductivity. Compos. A Appl. Sci. Manuf. 2012, 43, 1007–1015. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Jia, L.-C.; Zhang, G.; Yan, D.-X.; Li, Z.-M. A highly efficient and heat-resistant electromagnetic interference shielding carbon nanotube/poly (phenylene sulfide) composite via sinter molding. J. Mater. Chem. C 2018, 6, 10760–10766. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Castell, P.; Bespín-Gascón, S.; Ansón-Casaos, A.; Díez-Pascual, A.M.; Gómez-Fatou, M.A.; Benito, A.M.; Maser, W.K.; Martínez, M.T. Covalent functionalization of MWCNTs with poly (p-phenylene sulphide) oligomers: A route to the efficient integration through a chemical approach. J. Mater. Chem. 2012, 22, 21285–21297. [Google Scholar] [CrossRef]
- Park, M.; Park, J.H.; Yang, B.J.; Cho, J.; Kim, S.Y.; Jung, I. Enhanced interfacial, electrical, and flexural properties of polyphenylene sulfide composites filled with carbon fibers modified by electrophoretic surface deposition of multi-walled carbon nanotubes. Compos. A Appl. Sci. Manuf. 2018, 109, 124–130. [Google Scholar] [CrossRef]
- Ren, W.; Yang, Y.; Yang, J.; Duan, H.; Zhao, G.; Liu, Y. Multifunctional and corrosion resistant poly (phenylene sulfide)/Ag composites for electromagnetic interference shielding. Chem. Eng. J. 2021, 415, 129052–129063. [Google Scholar] [CrossRef]
- Kai, Z.H.; Gao, J.; Zhu, G.; Zhou, Z.; Xiang, H.; Qiu, T.; Zhu, M. Enhanced photo-stability polyphenylene sulfide fiber via incorporation of multi-walled carbon nanotubes using exciton quenching. Compos. A Appl. Sci. Manuf. 2020, 129, 105716–105723. [Google Scholar]
- Kim, M.; Lee, J.; Roh, H.; Kim, D.; Byeon, J.; Park, J. Effects of Covalent Functionalization of MWCNTs on the Thermal Properties and Non-Isothermal Crystallization Behaviors of PPS Composites. Polymers 2017, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Han, M.S.; Lee, Y.K.; Lee, H.S.; Yun, C.H.; Kim, W.N. Electrical, morphological and rheological properties of carbon nanotube composites with polyethylene and poly (phenylene sulfide) by melt mixing. Chem. Eng. Sci. 2009, 64, 4649–4656. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M. Enhancing the thermomechanical behaviour of poly (phenylene sulphide) based composites via incorporation of covalently grafted carbon nanotubes. Compos. A Appl. Sci. Manuf. 2013, 54, 10–19. [Google Scholar] [CrossRef]
- Nam, K.-H.; Kim, K.; Kim, S.G.; Lee, H.S.; Jung, H.; Yu, J.; Jang, S.G.; Ku, B.-C.; Moon, B.; You, N.-H. Sustainable production of reduced graphene oxide using elemental sulfur for multifunctional composites. Compos. B Eng. 2019, 176, 107236–107245. [Google Scholar] [CrossRef]
- Oh, Y.; Choi, H.K.; Jung, H.; Jin, J.-U.; Kim, Y.-K.; You, N.-H.; Ku, B.-C.; Kim, Y.; Yu, J. Analysis of the effect of organic solvent–sheet interfacial interaction on the exfoliation of sulfur-doped reduced graphene oxide sheets in a solvent system using molecular dynamics simulations. Phys. Chem. Chem. Phys. 2020, 22, 20665–20672. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, M.; You, N.-H.; Yu, J.; Yoo, H. High-flame retarding properties of polyacrylonitrile copolymer nanocomposites with synergistic effect of elemental sulfur-doped reduced graphene oxide and bio-derived catechol units. Compos. A Appl. Sci. Manuf. 2021, 21, 106477–106488. [Google Scholar] [CrossRef]
- Ren, P.-G.; Yan, D.-X.; Ji, X.; Chen, T.; Li, Z.-M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2011, 22, 055705–055712. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible Graphene Films via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef]
- Ortmann, F.; Schmidt, W.G.; Bechstedt, F. Attracted by Long-Range Electron Correlation: Adenine on Graphite. Phys. Rev. Lett. 2005, 95, 186101–186104. [Google Scholar] [CrossRef] [Green Version]
- Park, O.-K.; Hwang, J.-Y.; Goh, M.; Lee, J.H.; Ku, B.-C.; You, N.-H. Mechanically strong and multifunctional polyimide nanocomposites using amimophenyl functionalized graphene nanosheets. Macromolecules 2013, 46, 3505–3511. [Google Scholar] [CrossRef]
- Roy, S.; Das, T.; Zhanga, L.; Hu, X.M. Harnessing the maximum reinforcement of graphene oxide for poly (vinylidene fluoride) nanocomposites via polydopamine assisted novel surface modification. RSC Adv. 2016, 6, 69919–69929. [Google Scholar] [CrossRef]
- Jeon, J.; Choi, M.; Kim, S.B.; Seo, T.H.; Ku, B.-C.; Ryu, S.; Park, J.H.; Kim, Y.-K. Eggshell membrane hydrolysate as a multi-functional agent for synthesis of functionalized graphene analogue and its catalytic nanocomposites. J. Ind. Eng. Chem. 2021, 102, 233–240. [Google Scholar] [CrossRef]
- Gurzeda, B.; Kim, T.; Arsakay, M.; Choe, M.; Lee, S.; Lee, Z.; Min, S.; Ruoff, R. Electrochemical Formation of a Covalent-Ionic Stage-1 Graphite Intercalation Compound with Trifluoroacetic Acid. Chem. Mater. 2021, in press. [Google Scholar] [CrossRef]
- Fahey, D.R.; Ash, C.E. Mechanism of Poly (p-phenylene sulfide) Growth from p-Dichlorobenzene and Sodium Sulfide. Macromolecules 1991, 24, 4242–4249. [Google Scholar] [CrossRef]
- Rajan, C.R.; Ponrathnam, S.; Nadkarni, V.M. Poly (pheny1ene Sulfide): Polymerization Kinetics and Characterization. J. Appl. Polym. Sci. 1986, 32, 4479–4490. [Google Scholar] [CrossRef]
- Fahey, D.R.; Hensley, H.D.; Ash, C.E.; Senn, D.R. Poly (p-phenylene sulfide) Synthesis: A Step-Growth Polymerization with Unequal Step Reactivity. Macromolecules 1997, 30, 387–393. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Hay, A.S. A Facile Synthesis and the Polymerization of Macrocyclic 1,4-Phenylene Sulfide (PPS) Oligomers. Macromolecules 1996, 29, 5050–5053. [Google Scholar] [CrossRef]
- Hawkins, R.T. Chemistry of the Cure of Poly (p-phenylene sulfide). Macromolecules 1976, 9, 189–194. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Xiao, M.; Wang, S.J.; Ge, X.C.; Meng, Y.Z. Preparation and properties of electrically conductive PPS/expanded graphite nanocomposites. Compos. Sci. Technol. 2007, 67, 2528–2534. [Google Scholar] [CrossRef]
- Chae, B.-J.; Kim, D.H.; Jeong, I.-S.; Hahn, J.R.; Ku, B.-C. Electrical and Thermal Properties of Poly (p-phenylene sulfide) Reduced Graphite Oxide Nanocomposites. Carbon Lett. 2012, 13, 221–225. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Li, Z.; Cheng, B. Exfoliated graphite as a filler to improve poly (phenylene sulfide) electrical conductivity and mechanical properties. RSC Adv. 2012, 5, 13840–13849. [Google Scholar] [CrossRef]
- Gu, J.; Du, J.; Dang, J.; Geng, W.; Huab, S.; Zhang, Q. Thermal conductivities, mechanical and thermal properties of graphite nanoplatelets/polyphenylene sulfide composites. RSC Adv. 2014, 4, 22101–22105. [Google Scholar] [CrossRef]
- Jung, K.H.; Kim, H.J.; Kim, M.H.; Lee, J.-C. Preparation of Poly (phenylene sulfide)/Nylon 6 Grafted Graphene Oxide Nanocomposites with Enhanced Mechanical and Thermal Properties. Macromol. Res. 2020, 28, 241–248. [Google Scholar] [CrossRef]
- Ren, H.; Xu, D.; Yan, G.; Zhang, G.; Wang, X.; Long, S.; Yang, J. Effect of carboxylic polyphenylene sulfide on the micromechanical properties of polyphenylene sulfide/carbon fiber composites. Compos. Sci. Technol. 2017, 146, 65–72. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, J.T.; Li, X.; Dai, H. Solvothermal Reduction of Chemically Exfoliated Graphene Sheets. J. Am. Chem. Soc. 2009, 131, 9910–9911. [Google Scholar] [CrossRef] [PubMed]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.C.; Cha, K.; Hall, A.S.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R.B. A One-Step, Solvothermal Reduction Method for Producing Reduced Graphene Oxide Dispersions in Organic Solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Noh, Y.J.; Yu, J. Improved thermal conductivity of polymeric composites fabricated by solvent-free processing for the enhanced dispersion of nanofillers and a theoretical approach for composites containing multiple heterogeneities and geometrized nanofillers. Compos. Sci. Technol. 2014, 101, 79–85. [Google Scholar] [CrossRef]
- Kim, S.Y.; Noh, Y.J.; Yu, J. Thermal conductivity of graphene nanoplatelets filled composites fabricated by solvent-free processing for the excellent filler dispersion and a theoretical approach for the composites containing the geometrized fillers. Compos. A Appl. Sci. Manuf. 2015, 69, 219–225. [Google Scholar] [CrossRef]
- Kim, H.S.; Jang, J.; Yu, J.; Kim, S.Y. Thermal conductivity of polymer composites based on the length of multi-walled carbon nanotubes. Compos. B Eng. 2015, 79, 505–512. [Google Scholar] [CrossRef]











| Sample | Td5 (°C) | Char Yield (%) | Atomic Percent | C/O | IG/ID | d-Spacing (Å) | Electrical Conductivity (S/m) | ||
|---|---|---|---|---|---|---|---|---|---|
| C (%) | O (%) | S (%) | |||||||
| GO | 114.2 | 43.6 | 67.6 | 32.4 | - | 2.1 | 0.86 | 9.25 | - |
| SrGO | 193.9 | 69.2 | 76.4 | 20.6 | 3.0 | 3.7 | 1.08 | 3.72 | 442 |
| T-rGO | 438.2 | 85.9 | 88.8 | 11.2 | - | 8.0 | 1.04 | 3.46 | 430 |
| T-SrGO | 441.6 | 80.2 | 94.5 | 3.7 | 1.8 | 25.4 | 1.06 | 3.54 | 866 |
| Ratio | PPS | R1 | R5 | R10 | R20 | R30 | R40 | R50 | |
|---|---|---|---|---|---|---|---|---|---|
| PPS/GO | Td5 (°C) | 488.3 | 474.1 | 449.1 | 423.3 | 400.2 | 401.8 | 372.7 | 338.4 |
| Char Yield (%) | 42.9 | 42.5 | 41.5 | 43.2 | 43.1 | 46.4 | 43.4 | 53.2 | |
| Tm (°C) | 286.4 | 284.6 | 279.4 | 277.5 | 269.1 | 265.9 | 257.1 | 226.2 | |
| PPS/SrGO | Td5 (°C) | 488.3 | 484.0 | 450.3 | 392.8 | 368.7 | 367.4 | 262.1 | 376.8 |
| Char Yield (%) | 42.9 | 41.9 | 44.4 | 36.8 | 47.5 | 51.2 | 42.9 | 72.1 | |
| Tm (°C) | 286.4 | 284.6 | 280.9 | 273.7 | 269.2 | 250.6 | 234.4 | 244.5 | |
| Filler Contents (wt%) | 0 | 0.03 | 0.15 | 0.3 | |
|---|---|---|---|---|---|
| PPS/GO | Tensile Strength (MPa) | 60.19 ± 7.78 | 74.98 ± 7.51 | 71.41 ± 8.91 | 68.57 ± 6.45 |
| Modulus (GPa) | 3.89 ± 0.13 | 3.74 ± 0.10 | 3.82 ± 0.05 | 3.75 ± 0.09 | |
| Elongation (%) | 1.72 ± 0.61 | 2.40 ± 0.71 | 2.28 ± 0.47 | 2.23 ± 0.26 | |
| PPS/SrGO | Tensile Strength (MPa) | 60.19 ± 15.56 | 73.2 ± 11.88 | 72.04 ± 5.94 | 71.22 ± 13.58 |
| Modulus (GPa) | 3.89 ± 0.13 | 3.81 ± 0.08 | 3.84 ± 0.09 | 3.77 ± 0.11 | |
| Elongation (%) | 1.72 ± 0.61 | 2.28 ± 0.51 | 2.37 ± 0.33 | 2.30 ± 0.49 | |
| Filler Content (wt%) | 0 | 0.03 | 0.15 | 0.3 | |
|---|---|---|---|---|---|
| PPS/GO | Td5 (°C) | 483.9 | 483.2 | 482.5 | 482.2 |
| Char Yield (%) | 45.9 | 43.0 | 42.9 | 41.5 | |
| Tc (°C) | 199.1 | 220.3 | 216.4 | 208.2 | |
| Tm (°C) | 282.0 | 282.2 | 282.3 | 282.3 | |
| PPS/SrGO | Td5 (°C) | 483.9 | 483.7 | 484.9 | 483.8 |
| Char Yield (%) | 45.9 | 42.3 | 44.9 | 41.4 | |
| Tc (°C) | 199.1 | 211.9 | 219.7 | 223.3 | |
| Tm (°C) | 282.0 | 282.0 | 283.0 | 282.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.; Kim, J.; Oh, Y.; Yu, J.; Kim, S.-G.; Yoo, H.; Ryu, S.; You, N.-H.; Ku, B.-C. Surface Modification of Sulfur-Assisted Reduced Graphene Oxide with Poly(phenylene sulfide) for Multifunctional Nanocomposites. Polymers 2022, 14, 732. https://doi.org/10.3390/polym14040732
Choi M, Kim J, Oh Y, Yu J, Kim S-G, Yoo H, Ryu S, You N-H, Ku B-C. Surface Modification of Sulfur-Assisted Reduced Graphene Oxide with Poly(phenylene sulfide) for Multifunctional Nanocomposites. Polymers. 2022; 14(4):732. https://doi.org/10.3390/polym14040732
Chicago/Turabian StyleChoi, Minsik, Junghwan Kim, Yuna Oh, Jaesang Yu, Sung-Gi Kim, Heejoun Yoo, Seongwoo Ryu, Nam-Ho You, and Bon-Cheol Ku. 2022. "Surface Modification of Sulfur-Assisted Reduced Graphene Oxide with Poly(phenylene sulfide) for Multifunctional Nanocomposites" Polymers 14, no. 4: 732. https://doi.org/10.3390/polym14040732