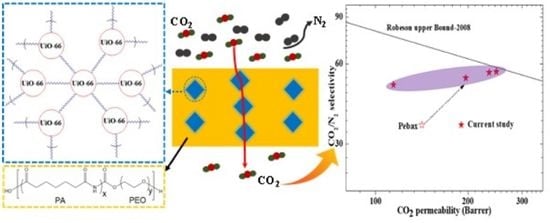
Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PIM-Grafted-MOF 1
2.3. Preparation of PIM-g-MOF (1)-Incorporated Pebax-Based MMMs, PIM-g-MOF-x
2.4. Characterization and Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of UiO-66-NH2(5) and Its PIM-Grafted-MOF Form, PIM-g-MOF (1)
3.2. Synthesis of PIM-g-MOF-x@Pebax
3.3. Characterization of the PIM-g-MOF-x MMMs
3.4. Thermal and Mechanical Properties of MMMs
3.5. Morphological Analyses by WAXD, SEM, and AFM
3.6. Gas Separation Performance of the Mixed Matrix Membranes
3.7. Permeability vs. Selectivity of the PIM-g-MOF-x
3.8. Anti-Aging Performance
3.9. Effect of Temperature on GasSeparation Performance
3.10. Pressure Effect on Gas Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, G.; Zou, X.; Sun, L.; Liu, B.; Wang, Z.; Zhang, P.; Zhu, G. Constructing Connected Paths between UiO-66 and PIM-1 to Improve Membrane CO2 Separation with Crystal-Like Gas Selectivity. Adv. Mater. 2019, 31, 1806853. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Cheng, J.; Hou, W.; Yang, C.; Yang, X.; Zhou, J. Bottom-up synthesis of two-dimensional composite via CuBDC-ns growth on multilayered MoS2 to boost CO2 permeability and selectivity in Pebax-based mixed matrix membranes. Sep. Purif. Technol. 2022, 282, 120007. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Hossain, I.; Nam, S.Y.; Rizzuto, C.; Barbieri, G.; Tocci, E.; Kim, T.H. PIM-polyimide multiblock copolymer-based membranes with enhanced CO2 separation performances. J. Membr. Sci. 2019, 574, 270–281. [Google Scholar] [CrossRef]
- Lin, R.B.; Xiang, S.; Zhou, W.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363. [Google Scholar] [CrossRef]
- Hossain, I.; al Munsur, A.Z.; Choi, O.; Kim, T.H. Bisimidazolium PEG-mediated crosslinked 6FDA-durene polyimide membranes for CO2 separation. Sep. Purif. Technol. 2019, 224, 180–188. [Google Scholar] [CrossRef]
- Hossain, I.; Kim, D.; al Munsur, A.Z.; Roh, J.M.; Park, H.B.; Kim, T.H. PEG/PPG-PDMS-Based Cross-Linked Copolymer Membranes Prepared by ROMP and in Situ Membrane Casting for CO2 Separation: An Approach to Endow Rubbery Materials with Properties of Rigid Polymers. ACS Appl. Mater. Interfaces 2020, 12, 27286–27299. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Hossain, I.; Park, S.; Husna, A.; Kim, Y.; Kim, H.; Kim, T.-H. PIM-PI-1 and Poly(ethylene glycol)/Poly(propylene glycol)-Based Mechanically Robust Copolyimide Membranes with High CO2 -Selectivity and an Anti-aging Property: A Joint Experimental–Computational Exploration. ACS Appl. Mater. Interfaces 2021, 13, 49890–49906. [Google Scholar] [CrossRef] [PubMed]
- Hossain, I.; Husna, A.; Chaemchuen, S.; Verpoort, F.; Kim, T.H. Cross-Linked Mixed-Matrix Membranes Using Functionalized UiO-66-NH2into PEG/PPG-PDMS-Based Rubbery Polymer for Efficient CO2 Separation. ACS Appl. Mater. Interfaces 2020, 12, 57916–57931. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Urban, J.J. Hydrogen-Bonded Polyimide/Metal-Organic Framework Hybrid Membranes for Ultrafast Separations of Multiple Gas Pairs. Adv. Funct. Mater. 2019, 29, 1903243. [Google Scholar] [CrossRef]
- Molavi, H.; Shojaei, A. Mixed-Matrix Composite Membranes Based on UiO-66-Derived MOFs for CO2 Separation. ACS Appl. Mater. Interfaces 2019, 11, 9448–9461. [Google Scholar] [CrossRef] [PubMed]
- Tien-Binh, N.; Rodrigue, D.; Kaliaguine, S. In-situ cross interface linking of PIM-1 polymer and UiO-66-NH2 for outstanding gas separation and physical aging control. J. Membr. Sci. 2018, 548, 429–438. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jones, J.T.A.; Hasell, T.; Cooper, A.I.; Bernardo, P.; Bazzarelli, F.; Clarizia, G.; Jansen, J.C. Nanoporous organic polymer/cage composite membranes. Angew. Chem.-Int. Ed. 2013, 52, 1253–1256. [Google Scholar] [CrossRef]
- Rangnekar, N.; Mittal, N.; Elyassi, B.; Caro, J.; Tsapatsis, M. Zeolite membranes—A review and comparison with MOFs. Chem. Soc. Rev. 2015, 44, 7128–7154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Ma, X.; Xiong, S.; Ye, Y.; Yao, Z.; Lin, Q.; Zhang, Z.; Xiang, S. Facile synthesis of oxidized activated carbons for high-selectivity and low-enthalpy CO2 capture from flue gas. New J. Chem. 2018, 42, 4495–4500. [Google Scholar] [CrossRef]
- Demakov, P.A.; Volynkin, S.S.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. A selenophene-incorporated metal–organic framework for enhanced CO2 uptake and adsorption selectivity. Molecules 2020, 25, 4396. [Google Scholar] [CrossRef] [PubMed]
- Bolotov, V.A.; Kovalenko, K.A.; Samsonenko, D.G.; Han, X.; Zhang, X.; Smith, G.L.; McCormick, L.J.; Teat, S.J.; Yang, S.; Lennox, M.J.; et al. Enhancement of CO2 Uptake and Selectivity in a Metal-Organic Framework by the Incorporation of Thiophene Functionality. Inorg. Chem. 2018, 57, 5074–5082. [Google Scholar] [CrossRef] [Green Version]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. Asymmetric Matrimid®/[Cu3(BTC)2] mixed-matrix membranes for gas separations. J. Membr. Sci. 2010, 362, 478–487. [Google Scholar] [CrossRef]
- Ordoñez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes. J. Membr. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Mixed-matrix membranes containing MOF-5 for gas separations. J. Membr. Sci. 2009, 328, 165–173. [Google Scholar] [CrossRef]
- Rodenas, T.; van Dalen, M.; García-Pérez, E.; Serra-Crespo, P.; Zornoza, B.; Kapteijn, F.; Gascon, J. Visualizing MOF mixed matrix membranes at the nanoscale: Towards structure-performance relationships in CO2/CH4 separation over NH2-MIL-53(Al)@PI. Adv. Funct. Mater. 2014, 24, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Zornoza, B.; Seoane, B.; Zamaro, J.M.; Téllez, C.; Coronas, J. Combination of MOFs and zeolites for mixed-matrix membranes. ChemPhysChem 2011, 12, 2781–2785. [Google Scholar] [CrossRef]
- Ma, L.; Svec, F.; Tan, T.; Lv, Y. Mixed Matrix Membrane Based on Cross-Linked Poly[(ethylene glycol) methacrylate] and Metal–Organic Framework for Efficient Separation of Carbon Dioxide and Methane. ACS Appl. Nano Mater. 2018, 1, 2808–2818. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Téllez, C.; de Vos, W.M.; Benes, N.E.; Konnertz, N.M.; Visser, T.; Semino, R.; et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Membr. Sci. 2018, 558, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Wang, Z.; Wang, M.; Qiao, Z.; Wang, J. High nanoparticles loadings mixed matrix membranes via chemical bridging-crosslinking for CO2 separation. J. Membr. Sci. 2019, 573, 455–464. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Caro, J.; Huang, A. A new UiO-66-NH2 based mixed-matrix membranes with high CO2/CH4 separation performance. Microporous Mesoporous Mater. 2019, 274, 203–211. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; He, S.; Bai, Y.; Shao, L. Interface manipulation of CO2-philic composite membranes containing designed UiO-66 derivatives towards highly efficient CO2 capture. J. Mater. Chem. A 2018, 6, 15064–15073. [Google Scholar] [CrossRef]
- Qian, Q.; Wu, A.X.; Chi, W.S.; Asinger, P.A.; Lin, S.; Hypsher, A.; Smith, Z.P. Mixed-Matrix Membranes Formed from Imide-Functionalized UiO-66-NH2 for Improved Interfacial Compatibility. ACS Appl. Mater. Interfaces 2019, 11, 31257–31269. [Google Scholar] [CrossRef] [Green Version]
- Cseri, L.; Hardian, R.; Anan, S.; Vovusha, H.; Schwingenschlögl, U.; Budd, P.M.; Sada, K.; Kokado, K.; Szekely, G. Bridging the interfacial gap in mixed-matrix membranes by nature-inspired design: Precise molecular sieving with polymer-grafted metal-organic frameworks. J. Mater. Chem. A 2021, 9, 23793–23801. [Google Scholar] [CrossRef]
- Husna, A.; Hossain, I.; Choi, O.; Lee, S.M.; Kim, T.H. Efficient CO2 Separation Using a PIM-PI-Functionalized UiO-66 MOF Incorporated Mixed Matrix Membrane in a PIM-PI-1 Polymer. Macromol. Mater. Eng. 2021, 306, 2100298. [Google Scholar] [CrossRef]
- Wang, H.; He, S.; Qin, X.; Li, C.; Li, T. Interfacial Engineering in Metal-Organic Framework-Based Mixed Matrix Membranes Using Covalently Grafted Polyimide Brushes. J. Am. Chem. Soc. 2018, 140, 17203–17210. [Google Scholar] [CrossRef]
- Katayama, Y.; Bentz, K.C.; Cohen, S.M. Defect-Free MOF-Based Mixed-Matrix Membranes Obtained by Corona Cross-Linking. ACS Appl. Mater. Interfaces 2019, 11, 13029–13037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal-organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Isanejad, M.; Mohammadi, T. Effect of amine modification on morphology and performance of poly (ether-block-amide)/fumed silica nanocomposite membranes for CO2/CH4 separation. Mater. Chem. Phys. 2018, 205, 303–314. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Y.; Wu, Y.; Zhang, B. Fabrication of Pebax/SAPO mixed matrix membranes for CO2/N2 separation. J. Appl. Polym. Sci. 2021, 138, 51336. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhai, L.; Ying, Y.; Wang, Y.; Liu, G.; Dong, J.; Ng, D.Z.L.; Khan, S.A.; Zhao, D. Highly efficient CO2 capture by mixed matrix membranes containing three-dimensional covalent organic framework fillers. J. Mater. Chem. A 2019, 7, 4549–4560. [Google Scholar] [CrossRef]
- Sarmadi, R.; Salimi, M.; Pirouzfar, V. The assessment of honeycomb structure UiO-66 and amino functionalized UiO-66 metal–organic frameworks to modify the morphology and performance of Pebax®1657-based gas separation membranes for CO2 capture applications. Environ. Sci. Pollut. Res. 2020, 27, 40618–40632. [Google Scholar] [CrossRef]
- Fam, W.; Mansouri, J.; Li, H.; Chen, V. Improving CO2 separation performance of thin film composite hollow fiber with Pebax®1657/ionic liquid gel membranes. J. Memb. Sci. 2017, 537, 54–68. [Google Scholar] [CrossRef]
- Azizi, N.; Isanejad, M.; Mohammadi, T.; Behbahani, R.M. Effect of TiO2 loading on the morphology and CO2/CH4 separation performance of PEBAX-based membranes. Front. Chem. Sci. Eng. 2019, 13, 517–530. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Jin, W.; Lee, K.-R.; Xu, N. Membranes with Fast and Selective Gas-Transport Channels of Laminar Graphene Oxide for Efficient CO2 Capture. Angew. Chem. 2015, 127, 588–592. [Google Scholar] [CrossRef]
- Gao, J.; Mao, H.; Jin, H.; Chen, C.; Feldhoff, A.; Li, Y. Functionalized ZIF-7/Pebax® 2533 mixed matrix membranes for CO2/N2 separation. Microporous Mesoporous Mater. 2020, 297, 110030. [Google Scholar] [CrossRef]
- Martínez-Izquierdo, L.; Malankowska, M.; Sánchez-Laínez, J.; Téllez, C.; Coronas, J. Poly(ether-block-amide) copolymer membrane for CO2/N2 separation: The influence of the casting solution concentration on its morphology, thermal properties and gas separation performance. R. Soc. Open Sci. 2019, 6, 190866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Xie, Q.; Ding, X.; Cai, R.; Tan, X.; Zhang, Y. Advanced mixed matrix membranes of Pebax embedded with amino acid ionic liquids@PIM core-shell composite nanoparticles for CO2 separation. Sep. Purif. Technol. 2021, 263, 118350. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax® MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Qin, Y.; Guo, R.; Zhang, J. Pebax–polydopamine microsphere mixed-matrix membranes for efficient CO2 separation. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar]
- Fan, L.; Kang, Z.; Shen, Y.; Wang, S.; Zhao, H.; Sun, H.; Hu, X.; Sun, H.; Wang, R.; Sun, D. Mixed Matrix Membranes Based on Metal-Organic Frameworks with Tunable Pore Size for CO2 Separation. Cryst. Growth Des. 2018, 18, 4365–4371. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Zulkifli, M.Y.; Li, H.; Zhang, Y.; Liang, W.; D’Alessandro, D.M.; Chen, V. Surface functionalized UiO-66/Pebax-based ultrathin composite hollow fiber gas separation membranes. J. Mater. Chem. A 2018, 6, 918–931. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Nasernejad, B.; Kargari, A. Cellulose acetate/nano-porous zeolite mixed matrix membrane for CO2 separation. Greenh. Gases Sci. Technol. 2015, 5, 291–304. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ahmadi, R.; Sinaei, M.; Kargari, A. Pebax-modified cellulose acetate membrane for CO2 /N2 separation. J. Membr. Sci. Res. 2019, 5, 25–32. [Google Scholar]
- Yong, W.F.; Li, F.Y.; Xiao, Y.C.; Li, P.; Pramoda, K.P.; Tong, Y.W.; Chung, T.S. Molecular engineering of PIM-1/Matrimid blend membranes for gas separation. J. Membr. Sci. 2012, 407–408, 47–57. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Li, K.; Ma, C.; Zhang, J.; Li, H.; Chang, X.; Zhu, L.; Xue, Q. Enhanced gas separation performance of Pebax mixed matrix membranes by incorporating ZIF-8 in situ inserted by multiwalled carbon nanotubes. Sep. Purif. Technol. 2020, 248, 117080. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Hua, K.; Deng, M. Poly(amide-6-b-ethylene oxide)/SAPO-34 mixed matrix membrane for CO2 separation. J. Energy Chem. 2014, 23, 227–234. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husna, A.; Hossain, I.; Jeong, I.; Kim, T.-H. Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer. Polymers 2022, 14, 655. https://doi.org/10.3390/polym14040655
Husna A, Hossain I, Jeong I, Kim T-H. Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer. Polymers. 2022; 14(4):655. https://doi.org/10.3390/polym14040655
Chicago/Turabian StyleHusna, Asmaul, Iqubal Hossain, Insu Jeong, and Tae-Hyun Kim. 2022. "Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer" Polymers 14, no. 4: 655. https://doi.org/10.3390/polym14040655