Functionalized Soybean Oil- and Vanillin-Based Dual Cure Photopolymerizable System for Light-Based 3D Structuring
Abstract
:1. Introduction
2. Materials and Methods
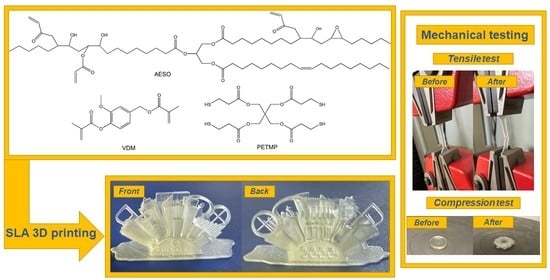
2.1. Materials
2.2. Preparation of Cross-Linked Polymer Specimens
2.3. Characterization Techniques
2.4. Real-Time Photorheometry
2.5. Two-Beam Initiation Threshold Experiment
2.6. SLA 3D Printing
3. Results and Discussion
3.1. Selection of Resin Composition
3.2. Monitoring of Photocuring Kinetics by Real-Time Photorheometry
3.3. Characterization of the Photocross-Linked Polymer Structure
3.4. Thermal Properties of Cross-Linked Polymer
3.5. Mechanical Characteristics of Cross-Linked Polymers
3.6. SLA 3D Printing
3.7. Determination of the Polymerization Threshold Using the 2-BIT Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skliutas, E.; Lebedevaite, M.; Kabouraki, E.; Baldacchini, T.; Ostrauskaite, J.; Vamvakaki, M.; Farsari, M.; Juodkazis, S.; Malinauskas, M. Polymerization mechanisms initiated by spatio-temporally confined light. Nanophotonics 2021, 10, 1211–1242. [Google Scholar] [CrossRef]
- Jonušauskas, L.; Gailevičius, D.; Rekštytė, S.; Baldacchini, T.; Juodkazis, S.; Malinauskas, M. Mesoscale laser 3D printing. Opt. Express 2019, 27, 15205–15221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, S.; Zhang, J.; Ali, I.; Li, X.; Liu, C. Recent trends in the development of biomass-based polymers from renewable resources and their environmental applications. J. Taiwan Inst. Chem. Eng. 2020, 115, 293–303. [Google Scholar] [CrossRef]
- Silva, J.; Grilo, L.; Gandini, A.; Lacerda, T. The Prospering of Macromolecular Materials Based on Plant Oils within the Blooming Field of Polymers from Renewable Resources. Polymers 2021, 13, 1722. [Google Scholar] [CrossRef]
- Zhanga, C.; Xuea, J.; Yanga, X.; Kea, Y.; Oua, R.; Wanga, Y.; Madboulyc, S.A.; Wang, Q. From plant phenols to novel bio-based polymers. Prog. Polym. Sci. 2022, 125, 101473. [Google Scholar] [CrossRef]
- Huang, X.; Ding, Z.; Wang, W.; Yan, X.; Zhou, Y.; Cai, Z. Synthesis and properties of porous materials from polymeric acrylated epoxidized soybean oil via an emulsion template. Ind. Crops Prod. 2022, 188, 115662. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Gineika, A.; Talacka, V.; Baltakys, K.; Ostrauskaite, J. Development and optical 3D printing of acrylated epox-idized soybean oil-based composites with functionalized calcium silicate hydrate filler derived from aluminium fluoride pro-duction waste. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106929. [Google Scholar] [CrossRef]
- Skliutas, E.; Lebedevaite, M.; Kasetaite, S.; Rekštytė, S.; Lileikis, S.; Ostrauskaite, J.; Malinauskas, M. A Bio-Based Resin for a Multi-Scale Optical 3D Printing. Sci. Rep. 2020, 10, 9758. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J.F.; Fernando, S.; Jagodzinski, K. Soy-based, high biorenewable content UV curable coatings. Prog. Org. Coat. 2011, 71, 98–109. [Google Scholar] [CrossRef]
- La Scala, J.; Wool, R.P. Property analysis of triglyceride-based thermosets. Polymer 2004, 46, 61–69. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin, a key-intermediate of biobased polymers. Eur. Polym. J. 2015, 68, 488–502. [Google Scholar] [CrossRef]
- Mohanraj, D.G.R.; Alagumuthu, M.; Subramaniam, P.; Bakthavachalam, D.; Arumugam, S.; Chellam, S. Antimicrobial effects of vanillin-based pyridyl-benzylidene-5-fluoroindolins. J. Heterocycl. Chem. 2021, 58, 1515–1524. [Google Scholar] [CrossRef]
- Polo, L.; de Greñu, B.D.; Della Bella, E.; Pagani, S.; Torricelli, P.; Vivancos, J.-L.; Ruiz-Rico, M.; Barat, J.M.; Aznar, E.; Martínez-Máñez, R.; et al. Antimicrobial activity of commercial calcium phosphate based materials functionalized with vanillin. Acta Biomater. 2018, 81, 293–303. [Google Scholar] [CrossRef]
- Grauzeliene, S.; Navaruckiene, A.; Skliutas, E.; Malinauskas, M.; Serra, A.; Ostrauskaite, J. Vegetable Oil-Based Thiol-Ene/Thiol-Epoxy Resins for Laser Direct Writing 3D Micro-/Nano-Lithography. Polymers 2021, 13, 872. [Google Scholar] [CrossRef] [PubMed]
- Navaruckiene, A.; Bridziuviene, D.; Raudoniene, V.; Rainosalo, E.; Ostrauskaite, J. Vanillin acrylate-based thermo-responsive shape memory antimicrobial photopolymers. Express Polym. Lett. 2022, 16, 279–295. [Google Scholar] [CrossRef]
- Roig, A.; Ramis, X.; De la Flor, S.; Serra, À. Dual-cured thermosets from glycydil methacrylate obtained by epoxy-amine reaction and methacrylate homopolymerization. React. Funct. Polym. 2021, 159, 104822. [Google Scholar] [CrossRef]
- Liu, J.; Miao, P.; Zhang, W.; Song, G.; Feng, J.; Leng, X.; Li, Y. Synthesis and characterization of interpenetrating polymer networks (IPNs) based on UV curable resin and blocked isocyanate/polyols. Polymer 2022, 256, 125254. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; Ramis, X.; Serra, À. State of the Art in Dual-Curing Acrylate Systems. Polymers 2018, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Nair, D.P.; Cramer, N.B.; Gaipa, J.C.; McBride, M.K.; Matherly, E.M.; McLeod, R.R.; Shandas, R.; Bowman, C.N. Two-Stage Reactive Polymer Network Forming Systems. Adv. Funct. Mater. 2012, 22, 1502–1510. [Google Scholar] [CrossRef]
- Green, W.A. Industrial Photoiniciators, A Technical Guide, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Ladika, D.; Noirbent, G.; Dumur, F.; Gigmes, D.; Mourka, A.; Barmparis, G.D.; Farsari, M.; Gray, D. Synthesis and application of triphenylamine-based aldehydes as photo-initiators for multi-photon lithography. Appl. Phys. A 2022, 128, 745. [Google Scholar] [CrossRef]
- Williams, H.E.; Diaz, C.; Padilla, G.; Hernandez, F.E.; Kuebler, S.M. Order of multiphoton excitation of sulfonium photo-acid generators used in photoresists based on SU-8. J. Appl. Phys. 2017, 121, 223104. [Google Scholar] [CrossRef]
- Yang, L.; Münchinger, A.; Kadic, M.; Hahn, V.; Mayer, F.; Blasco, E.; Barner-Kowollik, C.; Wegener, M. On the Schwarzschild Effect in 3D Two-Photon Laser Lithography. Adv. Opt. Mater. 2019, 7, 1901040. [Google Scholar] [CrossRef]
- Liaros, N. Far-field lithography through saturated resonance energy transfer. Opt. Lett. 2022, 47, 3327. [Google Scholar] [CrossRef] [PubMed]
- Tomova, Z.; Liaros, N.; Razo, S.A.G.; Wolf, S.M.; Fourkas, J.T. In situ measurement of the effective nonlinear absorption order in multiphoton photoresists. Laser Photon.-Rev. 2016, 10, 849–854. [Google Scholar] [CrossRef]
- Nguyen, D.T.T.; Tong, Q.C.; Ledoux-Rak, I.; Lai, N.D. One-step fabrication of submicrostructures by low one-photon absorption direct laser writing technique with local thermal effect. J. Appl. Phys. 2016, 119, 013101. [Google Scholar] [CrossRef]
- Zyla, G.; Surkamp, N.; Gurevich, E.L.; Esen, C.; Klehr, A.; Knigge, A.; Hofmann, M.R.; Ostendorf, A. Two-photon polymerization with diode lasers emitting ultrashort pulses with high repetition rate. Opt. Lett. 2020, 45, 4827. [Google Scholar] [CrossRef]
- Somers, P.; Liang, Z.; Johnson, J.E.; Boudouris, B.W.; Pan, L.; Xu, X. Rapid, continuous projection multi-photon 3D printing enabled by spatiotemporal focusing of femtosecond pulses. Light. Sci. Appl. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Kiefer, P.; Hahn, V.; Nardi, M.; Yang, L.; Blasco, E.; Barner-Kowollik, C.; Wegener, M. Sensitive Photoresists for Rapid Multiphoton 3D Laser Micro- and Nanoprinting. Adv. Opt. Mater. 2020, 8, 2000895. [Google Scholar] [CrossRef]
- Hahn, V.; Rietz, P.; Hermann, F.; Müller, P.; Barner-Kowollik, C.; Schlöder, T.; Wenzel, W.; Blasco, E.; Wegener, M. Light-sheet 3D microprinting via two-colour two-step absorption. Nat. Photon. 2022, 16, 784–791. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry, 1st ed.; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Ladika, M.; Farsari, M.; Gray, D. Two beam initiation threshold measurements of photo-initiators for laser writing of biocompatible 3D structures. In Proceedings of the 2019 Conference on Lasers and Electro-Optics Europe and European Quantum Electronics Conference, Munich, Germany, 23–27 June 2019. [Google Scholar]
- Lebedevaite, M.; Ostrauskaite, J. Influence of photoinitiator and temperature on photocross-linking kinetics of acrylated epoxidized soybean oil and properties of the resulting polymers. Ind. Crops Prod. 2021, 161, 113210. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, M.; Cochran, E.W.; Kessler, M.R. Biorenewable polymers based on acrylated epoxidized soybean oil and methacrylated vanillin. Mater. Today Commun. 2015, 5, 18–22. [Google Scholar] [CrossRef]
- Navaruckiene, A.; Skliutas, E.; Kasetaite, S.; Rekštytė, S.; Raudoniene, V.; Bridziuviene, D.; Malinauskas, M.; Ostrauskaite, J. Vanillin Acrylate-Based Resins for Optical 3D Printing. Polymers 2020, 12, 397. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.; Weisen, A.R.; Lee, Y.; Aplan, M.A.; Fenton, A.M.; Masucci, A.E.; Kempe, F.; Sommer, M.; Pester, C.W.; Colby, R.H.; et al. Glass transition temperature from the chemical structure of conjugated polymers. Nat. Commun. 2020, 11, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezger, T.G. The Rheology Handbook, 3rd ed.; Vincentz Network: Hanover, Germany, 2011. [Google Scholar]
- Gurr, M.; Mülhaupt, R. Rapid prototyping. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Edinburgh, UK, 2012; Volume 1, pp. 77–99. [Google Scholar]
- Navaruckiene, A.; Kasetaite, S.; Ostrauskaite, J. Vanillin-based thiol-ene systems as photoresins for optical 3D printing. Rapid Prototyp. J. 2019, 26, 402–408. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Ostrauskaite, J.; Skliutas, E.; Malinauskas, M. Photocross-linked polymers based on plant-derived monomers for potential application in optical 3D printing. J. Appl. Polym. Sci. 2019, 137, 48708. [Google Scholar] [CrossRef]
- Vyas, A.; Garg, V.; Ghosh, S.B.; Bandyopadhyay-Ghosh, S. Photopolymerizable resin-based 3D printed biomedical composites: Factors affecting resin viscosity. Mater. Today Proc. 2022, 62, 1435–1439. [Google Scholar] [CrossRef]
- Zandrini, T.; Liaros, N.; Jiang, L.J.; Lu, Y.F.; Fourkas, J.T.; Osellame, R.; Baldacchini, T. Effect of the resin viscosity on the writing properties of two-photon polymerization. Opt. Mater. Express 2019, 9, 2601–2616. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sereikaite, V.; Navaruckiene, A.; Jaras, J.; Skliutas, E.; Ladika, D.; Gray, D.; Malinauskas, M.; Talacka, V.; Ostrauskaite, J. Functionalized Soybean Oil- and Vanillin-Based Dual Cure Photopolymerizable System for Light-Based 3D Structuring. Polymers 2022, 14, 5361. https://doi.org/10.3390/polym14245361
Sereikaite V, Navaruckiene A, Jaras J, Skliutas E, Ladika D, Gray D, Malinauskas M, Talacka V, Ostrauskaite J. Functionalized Soybean Oil- and Vanillin-Based Dual Cure Photopolymerizable System for Light-Based 3D Structuring. Polymers. 2022; 14(24):5361. https://doi.org/10.3390/polym14245361
Chicago/Turabian StyleSereikaite, Vilte, Aukse Navaruckiene, Justinas Jaras, Edvinas Skliutas, Dimitra Ladika, David Gray, Mangirdas Malinauskas, Vaidas Talacka, and Jolita Ostrauskaite. 2022. "Functionalized Soybean Oil- and Vanillin-Based Dual Cure Photopolymerizable System for Light-Based 3D Structuring" Polymers 14, no. 24: 5361. https://doi.org/10.3390/polym14245361