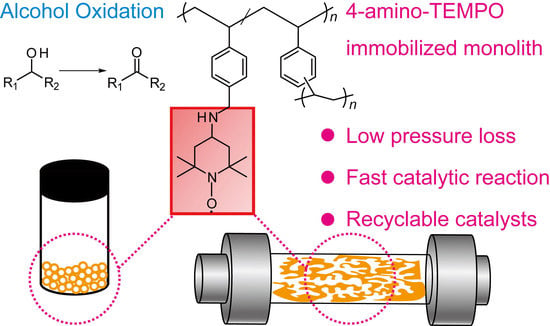
4-Amino-TEMPO-Immobilized Polymer Monolith: Preparations, and Recycling Performance of Catalyst for Alcohol Oxidation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Preparation of Poly (4-Chloromethyl-Styrene-co-DVB) Monolith (Cl−Monolith)
2.4. Preparation of 4-Amino-TEMPO-Immobilized Monolith (Monolith)
2.5. Permeabilities of Continuous-Flow Reactor
2.6. Flow Behavior Analysis
2.7. Oxidation Reaction with Immobilized Catalyst
3. Results and Discussion
3.1. Preparation of Monolith (4-Amino-TEMPO-Immobilized Monolith)
3.2. Batch Oxidation Reaction
3.3. Flow Oxidation Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ley, S.V.; Chen, Y.; Robinson, A.; Otter, B.; Godineau, E.; Battilocchio, C. A Comment on Continuous Flow Technologies within the Agrochemical Industry. Org. Process Res. Dev. 2021, 25, 713–720. [Google Scholar] [CrossRef]
- Seto, M.; Masada, S.; Usutani, H.; Cork, D.G.; Fukuda, K.; Kawamoto, T. Application of Continuous Flow-Flash Chemistry to Scale-up Synthesis of 5-Cyano-2-formylbenzoic Acid. Org. Process Res. Dev. 2019, 23, 1420–1428. [Google Scholar] [CrossRef]
- Yoo, W.-J.; Ishitani, H.; Saito, Y.; Laroche, B.; Kobayashi, S. Reworking Organic Synthesis for the Modern Age: Synthetic Strategies Based on Continuous-Flow Addition and Condensation Reactions with Heterogeneous Catalysts. J. Org. Chem. 2020, 85, 5132–5145. [Google Scholar] [CrossRef] [PubMed]
- Makgwane, R.R.; Ray, S.S. Synthesis of Nanomaterials by Continuous-Flow Microfluidics: A Review. J. Nanosci. Nanotech. 2014, 14, 1338–1363. [Google Scholar] [CrossRef]
- Kirschning, A.; Solodenko, W.; Mennecke, K. Combining Enabling Techniques in Organic Synthesis: Continuous Flow Processes with Heterogenized Catalysts. Chemistry 2006, 12, 5972–5990. [Google Scholar] [CrossRef]
- Kobayashi, S. Immobilized Catalysts in Combinatorial Chemistry. Curr. Opin. Chem. Biol. 2000, 4, 338–345. [Google Scholar] [CrossRef]
- Munirathinam, R.; Huskens, J.; Verboom, W. Supported Catalysis in Continuous-Flow Microreactors. Adv. Synth. Catal. 2015, 357, 1093–1123. [Google Scholar] [CrossRef]
- Rebrov, E.V.; Berenguer-Murcia, A.; Skelton, H.E.; Johnson, B.F.; Wheatley, A.E.; Schouten, J.C. Capillary Microreactors Wall-Coated with Mesoporous Titania Thin Film Catalyst Supports. Lab Chip 2009, 9, 503–506. [Google Scholar] [CrossRef] [Green Version]
- Losey, M.W.; Schmidt, M.A.; Jensen, K.F. Microfabricated Multiphase Packed-Bed Reactors: Characterization of Mass Transfer and Reactions. Ind. Eng. Chem. Res. 2001, 40, 2555–2562. [Google Scholar] [CrossRef]
- Siouffi, A.M. About the C Term in the van Deemter’s Equation of Plate Height in Monoliths. J. Chromatogr. A 2006, 1126, 86–94. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, Y.; Iwai, T.; Sawamura, M.; Miura, Y. Polystyrene-Cross-Linking Triphenylphosphine on a Porous Monolith: Enhanced Catalytic Activity for Aryl Chloride Cross-Coupling in Biphasic Flow. Ind. Eng. Chem. Res. 2020, 59, 15179–15187. [Google Scholar] [CrossRef]
- Matsumoto, H.; Seto, H.; Akiyoshi, T.; Shibuya, M.; Hoshino, Y.; Miura, Y. Macroporous Gel with a Permeable Reaction Platform for Catalytic Flow Synthesis. ACS Omega 2017, 2, 8796–8802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, H.; Hoshino, Y.; Iwai, T.; Sawamura, M.; Miura, Y. Polystyrene-Supported PPh3 in Monolithic Porous Material: Effect of Cross-Linking Degree on Coordination Mode and Catalytic Activity in Pd-Catalyzed C−C Cross-Coupling of Aryl Chlorides. ChemCatChem 2020, 12, 4034–4037. [Google Scholar] [CrossRef]
- Nonaka, S.; Matsumoto, H.; Nagao, M.; Hoshino, Y.; Miura, Y. Investigation of the Effect of Microflow Reactor Diameter on Condensation Reactions in L-proline-Immobilized Polymer Monoliths. React. Chem. Eng. 2022, 7, 55–60. [Google Scholar] [CrossRef]
- Yang, H.; Shi, Q.; Tian, B.; Xie, S.; Zhang, F.; Yan, Y.; Zhao, D. A Fast Way for Preparing Crack-Free Mesostructured Silica Monolith. Chem. Mater. 2003, 15, 536–541. [Google Scholar] [CrossRef]
- Hormann, K.; Müllner, T.; Bruns, S.; Höltzel, A.; Tallarek, U. Morphology and Separation Efficiency of a New Generation of Analytical Silica Monoliths. J. Chromatogr. A 2012, 1222, 46–58. [Google Scholar] [CrossRef]
- Krause, J.O.; Lubbad, S.; Nuyken, O.; Buchmeiser, M.R. Monolith- and Silica-Supported Carboxylate-Based Grubbs–Herrmann-Type Metathesis Catalysts. Adv. Synth. Catal. 2003, 345, 996–1004. [Google Scholar] [CrossRef]
- Rohr, T.; Hilder, E.F.; Donovan, J.J.; Svec, F.; Frechet, J.M. Photografting and the Control of Surface Chemistry in Three-Dimensional Porous Polymer Monoliths. Macromolecules 2003, 36, 1677–1684. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, T.B.; Rohr, T.; Hilder, E.F.; Peterson, D.S.; Yi, M.; Svec, F.; Fréchet, J.M. Fabrication of Porous Polymer Monoliths Covalently Attached to the Walls of Channels in Plastic Microdevices. Electrophoresis 2003, 24, 3689–3693. [Google Scholar] [CrossRef]
- Okada, K.; Nandi, M.; Maruyama, J.; Oka, T.; Tsujimoto, T.; Kondoh, K.; Uyama, H. Fabrication of Mesoporous Polymer Monolith: A Template-Free Approach. Chem. Commun. 2011, 47, 7422–7424. [Google Scholar] [CrossRef]
- Xin, Y.; Fujimoto, T.; Uyama, H. Facile Fabrication of Polycarbonate Monolith by Non-Solvent Induced Phase Separation Method. Polymer 2012, 53, 2847–2853. [Google Scholar] [CrossRef]
- Schulze, J.S.; Migenda, J.; Becker, M.; Schuler, S.M.; Wende, R.C.; Schreiner, P.R.; Smarsly, B.M. Synthesis and Testing of Functional Mesoporous Silica Nanoparticles for Removal of Cr(VI) Ions From Water. J. Mat. Chem. A 2020, 8, 4107–4117. [Google Scholar] [CrossRef]
- Mogharabi-Manzari, M.; Kiani, M.; Aryanejad, S.; Imanparast, S.; Amini, M.; Faramarzi, M.A. A Magnetic Heterogeneous Biocatalyst Composed of Immobilized Laccase and 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO) for Green One-Pot Cascade Synthesis of 2-Substituted Benzimidazole and Benzoxazole Derivatives under Mild Reaction Conditions. Adv. Synth. Catal. 2018, 360, 3563–3571. [Google Scholar] [CrossRef]
- Okuno, Y.; Kitagawa, Y.; Kamiya, S.; Hasegawa, A.; Kawashima, T.; Otani, K.; Aoki, S.; Kanno, J.; Isomura, S. Triphasic Continuous-Flow Oxidation System for Alcohols Utilizing Graft-Polymer-Supported TEMPO. Asian J. Org. Chem. 2018, 7, 1071–1074. [Google Scholar] [CrossRef]
- Meyer, U.; Svec, F.; Fréchet, J.M.; Hawker, C.J.; Irgum, K. Use of Stable Free Radicals for the Sequential Preparation and Surface Grafting of Functionalized Macroporous Monoliths. Macromolecules 2000, 33, 7769–7775. [Google Scholar] [CrossRef]
- Urban, J.; Eeltink, S.; Jandera, P.; Schoenmakers, P.J. Characterization of polymer-based monolithic capillary columns by inverse size-exclusion chromatography and mercury-intrusion porosimetry. J. Chromatogr. A 2008, 1182, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gelotte, B. Studies on Gel Filtration: Sorption Properties of the Bed Material Sephadex. J. Chromatogr. A 1960, 3, 330–342. [Google Scholar] [CrossRef]
- Knox, J.H.; Scott, H.P. Theoretical Models for Size-Exclusion Chromatography and Calculation of Pore Size Distribution from Size-Exclusion Chromatography Data. J. Chromatogr. A 1984, 316, 311–332. [Google Scholar] [CrossRef]






| Material | DVB [wt %] | Specific Surface Area [m2 g−1] a | Average Pore Diameter [μm] a | Porosity [%] a | 4-Amino-TEMPO [mmol/g] b | Permeability [m2] c |
|---|---|---|---|---|---|---|
| Monolith−1 | 20 | 0.85 | 8.8 | 65 | 0.53 | 5.7 × 10−12 |
| Monolith−2 | 30 | 0.74 | 8.4 | 61 | 0.36 | 4.1 × 10−13 |
| Monolith−3 | 40 | 18 | 0.34 | 59 | 0.33 | 7.3 × 10−14 |
| Monolith−4 | 50 | 29 | 0.23 | 64 | 0.24 | 3.5 × 10−14 |
| Substrate | Product | The Yield of 4 min [%] | The Yield of 8 min [%] |
|---|---|---|---|
Benzyl alcohol | Benzaldehyde | 48 | 52 |
2-Phenylethanol | Phenylacetaldehyde | 18 | 48 |
(+/−)-1-Phenylethanol | Acetophenone | 22 | 39 |
4-Nitrobenzyl alcohol | 4-Nitrobenzaldehyde | 35 | 30 |
2-Methoxybenzyl alcohol | 2-Methoxybenzaldehyde | 59 | 59 |
4-Methoxybenzyl alcohol | 4-Methoxybenzaldehyde | 62 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imoto, T.; Matsumoto, H.; Nonaka, S.; Shichijo, K.; Nagao, M.; Shimakoshi, H.; Hoshino, Y.; Miura, Y. 4-Amino-TEMPO-Immobilized Polymer Monolith: Preparations, and Recycling Performance of Catalyst for Alcohol Oxidation. Polymers 2022, 14, 5123. https://doi.org/10.3390/polym14235123
Imoto T, Matsumoto H, Nonaka S, Shichijo K, Nagao M, Shimakoshi H, Hoshino Y, Miura Y. 4-Amino-TEMPO-Immobilized Polymer Monolith: Preparations, and Recycling Performance of Catalyst for Alcohol Oxidation. Polymers. 2022; 14(23):5123. https://doi.org/10.3390/polym14235123
Chicago/Turabian StyleImoto, Tomoki, Hikaru Matsumoto, Seiya Nonaka, Keita Shichijo, Masanori Nagao, Hisashi Shimakoshi, Yu Hoshino, and Yoshiko Miura. 2022. "4-Amino-TEMPO-Immobilized Polymer Monolith: Preparations, and Recycling Performance of Catalyst for Alcohol Oxidation" Polymers 14, no. 23: 5123. https://doi.org/10.3390/polym14235123