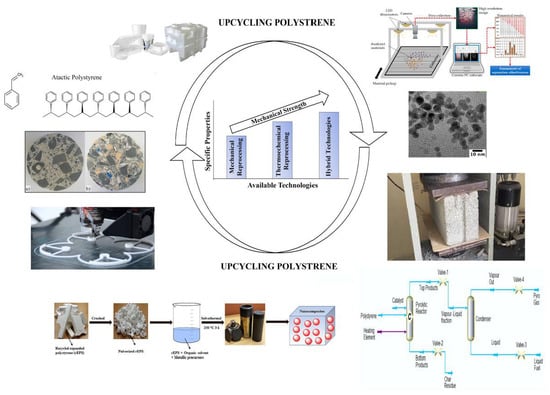
Upcycling Polystyrene
Abstract
:1. Introduction
1.1. Polystyrene
1.1.1. Chemistry and Synthesis
1.1.2. Polystyrene Grades and Applications
- High-impact polystyrene (HIPS):
- Oriented polystyrene (OPS)
- Expanded polystyrene (EPS)
- Styrenic copolymers
- Brominated polystyrene:
2. Dealing with PS Wastes
2.1. Mechanical Methods
2.1.1. Conventional Mechanical Reprocessing
2.1.2. Landfilling
2.2. Thermochemical Methods
2.2.1. Conventional Incineration
2.2.2. Conventional Pyrolysis
2.2.3. Microwave-Assisted Pyrolysis
2.2.4. Conventional Gasification
2.2.5. Hydrogenolysis
2.2.6. Catalytic Cracking
2.2.7. Dissolution/Precipitation
2.2.8. Depolymerization
2.2.9. Photo-Reforming
2.2.10. Sustainable PS-Based Material Design
2.2.11. Use of Biodegradable Polymers
2.2.12. Biochemical Conversion
2.3. Assessments of PS Recycling Methods
3. Toughening Approaches of Recycled PS as an Upcycling Strategy
3.1. Use of Compatibilizers
3.2. Addition of Tyre Crumbs or Rubber
3.3. Ternary Blends
3.4. Ionic-Crosslinking
3.5. Addition of Polymers, Organic and Inorganic Fillers
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Larrain, M.; Van Passel, S.; Thomassen, G.; Van Gorp, B.; Nhu, T.T.; Huysveld, S.; Van Geem, K.M.; De Meester, S.; Billen, P. Techno-Economic Assessment of Mechanical Recycling of Challenging Post-Consumer Plastic Packaging Waste. Resour. Conserv. Recycl. 2021, 170, 105607. [Google Scholar] [CrossRef]
- Peinado, F.; Aldas, M.; López-Martínez, J.; Samper, M.D. Recycling of the Styrene Fraction from Post-Consumer Waste. Mater. Tehnol. 2020, 54, 725–730. [Google Scholar] [CrossRef]
- Hirayama, D.; Nunnenkamp, L.A.; Braga, F.H.G.; Saron, C. Enhanced Mechanical Properties of Recycled Blends Acrylonitrile–Butadiene–Styrene/High–Impact Polystyrene from Waste Electrical and Electronic Equipment Using Compatibilizers and Virgin Polymers. J. Appl. Polym. Sci. 2022, 139, 51873. [Google Scholar] [CrossRef]
- Civancik-Uslu, D.; Nhu, T.T.; Van Gorp, B.; Kresovic, U.; Larrain, M.; Billen, P.; Ragaert, K.; De Meester, S.; Dewulf, J.; Huysveld, S. Moving from Linear to Circular Household Plastic Packaging in Belgium: Prospective Life Cycle Assessment of Mechanical and Thermochemical Recycling. Resour. Conserv. Recycl. 2021, 171, 105633. [Google Scholar] [CrossRef]
- Zhao, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.J.; Ozcan, S. Plastic Waste Upcycling toward a Circular Economy. Chem. Eng. J. 2022, 428, 131928. [Google Scholar] [CrossRef]
- Lu, C.; Yu, X.; Guo, S. The Mechanism of Ultrasonic Improvement of Weld Line Strength of Injection Molded Polystyrene and Polystyrene/Polyethylene Blend Parts. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1520–1530. [Google Scholar] [CrossRef]
- Zhenova, A.; Pellis, A.; Milescu, R.A.; McElroy, C.R.; White, R.J.; Clark, J.H. Solvent Applications of Short-Chain Oxymethylene Dimethyl Ether Oligomers. ACS Sustain. Chem. Eng. 2019, 7, 14834–14840. [Google Scholar] [CrossRef]
- Saalman, E. Infrared Spectroscopic Study of Polymers Exposed to Ethylene Oxide. Biomaterials 1985, 6, 225–230. [Google Scholar] [CrossRef]
- Croonenborghs, B.; Smith, M.A.; Strain, P. X-ray versus Gamma Irradiation Effects on Polymers. Radiat. Phys. Chem. 2007, 76, 1676–1678. [Google Scholar] [CrossRef]
- Lin, C.-C.; Ming, L.-J.; Lee, C.-C.; Lee, S. EPR Kinetics in Irradiated Syndiotactic Polystyrene at Elevated Temperatures. Polymer 2008, 49, 3987–3992. [Google Scholar] [CrossRef]
- Sastri, V.R. Commodity Thermoplastics. In Plastics in Medical Devices; Elsevier: Amsterdam, The Netherlands, 2014; pp. 73–120. [Google Scholar]
- Aciu, C.; Manea, D.L.; Molnar, L.M.; Jumate, E. Recycling of Polystyrene Waste in the Composition of Ecological Mortars. Procedia Technol. 2015, 19, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Demirors, M. Styrene polymers and copolymers. In Applied Polymer Science: 21st Century; Elsevier: Amsterdam, The Netherlands, 2000; pp. 93–106. [Google Scholar]
- Beghetto, V.; Sole, R.; Buranello, C.; Al-Abkal, M.; Facchin, M. Recent Advancements in Plastic Packaging Recycling: A Mini-Review. Materials 2021, 14, 4782. [Google Scholar] [CrossRef] [PubMed]
- Selina, M.; Markus, B.; Daniel, S.; Renato, S. Wet-Mechanical Processing of a Plastic-Rich Two-Dimensional-Fraction from Mixed Wastes for Chemical Recycling. Waste Manag. Res. J. Sustain. Circ. Econ. 2021, 39, 731–743. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Silvenius, F.; Katajajuuri, J.-M.; Grönman, K.; Soukka, R.; Koivupuro, H.-K.; Virtanen, Y. Role of Packaging in LCA of Food Products. In Towards Life Cycle Sustainability Management; Springer: Dordrecht, The Netherlands, 2011; pp. 359–370. [Google Scholar]
- El Bhilat, H.; Hachim, A.; Salmi, H.; Mabchour, H.; El Had, K. Characterization and Processability of Post-Consumer, Double-Recycled High Impact Polystyrene from Disposable Cups. Mater. Today Proc. 2021, 45, 7264–7270. [Google Scholar] [CrossRef]
- Signoret, C.; Caro-Bretelle, A.-S.; Lopez-Cuesta, J.-M.; Ienny, P.; Perrin, D. Alterations of Plastics Spectra in MIR and the Potential Impacts on Identification towards Recycling. Resour. Conserv. Recycl. 2020, 161, 104980. [Google Scholar] [CrossRef]
- Rybarczyk, D.; Jędryczka, C.; Regulski, R.; Sędziak, D.; Netter, K.; Czarnecka-Komorowska, D.; Barczewski, M.; Barański, M. Assessment of the Electrostatic Separation Effectiveness of Plastic Waste Using a Vision System. Sensors 2020, 20, 7201. [Google Scholar] [CrossRef] [PubMed]
- Moroni, M.; Mei, A. Characterization and Separation of Traditional and Bio-Plastics by Hyperspectral Devices. Appl. Sci. 2020, 10, 2800. [Google Scholar] [CrossRef] [Green Version]
- Almishal, S.S.I.; Mohamed, T.A.; Shazly, M. Experimental and Statistical Study of the Effect of Temperature and Waste Ratio on the Mechanical Properties and Cost of Polystyrene Polypropylene Plastic Blends. Heliyon 2020, 6, e04166. [Google Scholar] [CrossRef]
- Uttaravalli, A.N.; Dinda, S.; Gidla, B.R. Scientific and Engineering Aspects of Potential Applications of Post-Consumer (Waste) Expanded Polystyrene: A Review. Process Saf. Environ. Prot. 2020, 137, 140–148. [Google Scholar] [CrossRef]
- Maafa, I. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Ponnusamy, V.K.; Pugazhendhi, A.; Nižetić, S.; Praveenkumar, T.R. Production and Utilization of Pyrolysis Oil from Solidplastic Wastes: A Review on Pyrolysis Process and Influence of Reactors Design. J. Environ. Manag. 2022, 302, 114046. [Google Scholar] [CrossRef] [PubMed]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. ChemistryOpen 2021, 10, 1202–1226. [Google Scholar] [CrossRef] [PubMed]
- Inayat, A.; Klemencova, K.; Grycova, B.; Sokolova, B.; Lestinsky, P. Thermo-Catalytic Pyrolysis of Polystyrene in Batch and Semi-Batch Reactors: A Comparative Study. Waste Manag. Res. J. Sustain. Circ. Econ. 2021, 39, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Pinto, F.; Mata, R.; Marques, P.A.A.P.; Paradela, F.; Costa, L.C. Validation of the Application of the Pyrolysis Process for the Treatment and Transformation of Municipal Plastic Wastes. Chem. Eng. Trans. 2021, 86, 859–864. [Google Scholar]
- Leadbeater, N. Microwave Heating as a Tool for Sustainable Chemistry; Leadbeater, N.E., Ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439812709. [Google Scholar]
- Díaz-Ortiz, Á.; Prieto, P.; de la Hoz, A. A Critical Overview on the Effect of Microwave Irradiation in Organic Synthesis. Chem. Rec. 2019, 19, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Zhang, Y.; Liu, T.; Fu, W.; Li, B. Microwave-Assisted Pyrolysis of Polystyrene for Aviation Oil Production. J. Anal. Appl. Pyrolysis 2022, 162, 105425. [Google Scholar] [CrossRef]
- Kumar, D.; Rani, S.; Nandan, B.; Srivastava, R.K. Nonpolar Graphene Quantum Dot-Based Hydrophobic Coating from Microwave-Assisted Treatment of Styrofoam Waste. ACS Sustain. Chem. Eng. 2022, 10, 1070–1077. [Google Scholar] [CrossRef]
- Rex, P.; Masilamani, I.P.; Miranda, L.R. Microwave Pyrolysis of Polystyrene and Polypropylene Mixtures Using Different Activated Carbon from Biomass. J. Energy Inst. 2020, 93, 1819–1832. [Google Scholar] [CrossRef]
- Terapalli, A.; Kamireddi, D.; Sridevi, V.; Tukarambai, M.; Suriapparao, D.V.; Rao, C.S.; Gautam, R.; Modi, P.R. Microwave-Assisted in-Situ Catalytic Pyrolysis of Polystyrene: Analysis of Product Formation and Energy Consumption Using Machine Learning Approach. Process Saf. Environ. Prot. 2022, 166, 57–67. [Google Scholar] [CrossRef]
- Arshad, H.; Sulaiman, S.A.; Hussain, Z.; Moni, M.N.Z. Effect of Reaction Time and Microwave Power on Coil Temperature during Microwave-Metal Interaction Pyrolysis of Plastics. IOP Conf. Ser. Mater. Sci. Eng. 2020, 863, 012007. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-Pyrolysis of Waste Plastic and Solid Biomass for Synergistic Production of Biofuels and Chemicals-A Review. Prog. Energy Combust. Sci. 2021, 84, 100899. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Yerrayya, A.; Nagababu, G.; Guduru, R.K.; Kumar, T.H. Recovery of Renewable Aromatic and Aliphatic Hydrocarbon Resources from Microwave Pyrolysis/Co-Pyrolysis of Agro-Residues and Plastics Wastes. Bioresour. Technol. 2020, 318, 124277. [Google Scholar] [CrossRef] [PubMed]
- Suriapparao, D.V.; Sridevi, V.; Ramesh, P.; Sankar Rao, C.; Tukarambai, M.; Kamireddi, D.; Gautam, R.; Dharaskar, S.A.; Pritam, K. Synthesis of Sustainable Chemicals from Waste Tea Powder and Polystyrene via Microwave-Assisted in-Situ Catalytic Co-Pyrolysis: Analysis of Pyrolysis Using Experimental and Modeling Approaches. Bioresour. Technol. 2022, 362, 127813. [Google Scholar] [CrossRef]
- Ramzan, F.; Shoukat, B.; Naz, M.Y.; Shukrullah, S.; Ahmad, F.; Naz, I.; Makhlouf, M.M.; Farooq, M.U.; Kamran, K. Single Step Microwaves Assisted Catalytic Conversion of Plastic Waste into Valuable Fuel and Carbon Nanotubes. Thermochim. Acta 2022, 715, 179294. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Hemanth Kumar, T.; Reddy, B.R.; Yerrayya, A.; Srinivas, B.A.; Sivakumar, P.; Prakash, S.R.; Sankar Rao, C.; Sridevi, V.; Desinghu, J. Role of ZSM5 Catalyst and Char Susceptor on the Synthesis of Chemicals and Hydrocarbons from Microwave-Assisted in-Situ Catalytic Co-Pyrolysis of Algae and Plastic Wastes. Renew. Energy 2022, 181, 990–999. [Google Scholar] [CrossRef]
- Ertem, S.P.; Onuoha, C.E.; Wang, H.; Hillmyer, M.A.; Reineke, T.M.; Lodge, T.P.; Bates, F.S. Hydrogenolysis of Linear Low-Density Polyethylene during Heterogeneous Catalytic Hydrogen–Deuterium Exchange. Macromolecules 2020, 53, 6043–6055. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Yang, Y.; Liu, Y.; Li, Z. A Metal-Free Approach for the Hydrogenolysis of Unactivated C(Aryl)–C(Alkyl) Bonds; Preprint (Version 1) Available at Research Square; Springer Nature: Berlin, Germany, 2021. [Google Scholar] [CrossRef]
- Yao, L.; King, J.; Wu, D.; Ma, J.; Li, J.; Xie, R.; Chuang, S.S.C.; Miyoshi, T.; Peng, Z. Non-Thermal Plasma-Assisted Rapid Hydrogenolysis of Polystyrene to High Yield Ethylene. Nat. Commun. 2022, 13, 885. [Google Scholar] [CrossRef]
- Thambiyapillai, S.; Ramanujam, M. An Experimental Investigation and Aspen HYSYS Simulation of Waste Polystyrene Catalytic Cracking Process for the Gasoline Fuel Production. Int. J. Renew. Energy Dev. 2021, 10, 891–900. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Lu, H.; Huang, Z. Recycling of Phosphorus-Containing Plastic Based on the Dual Effects of Switchable Hydrophilicity Solvents. Chemosphere 2020, 259, 127402. [Google Scholar] [CrossRef]
- John, G.; Nagarajan, S.; Vemula, P.K.; Silverman, J.R.; Pillai, C.K.S. Natural Monomers: A Mine for Functional and Sustainable Materials—Occurrence, Chemical Modification and Polymerization. Prog. Polym. Sci. 2019, 92, 158–209. [Google Scholar] [CrossRef]
- Jimenez-Francisco, M.; Flores-Johnson, E.A.; Carrillo, J.G. Effect of Recycled Polystyrene/Limonene Coating on the Mechanical Properties of Kraft Paper: A Comparative Study with Commercial Coatings. J. Polym. Environ. 2020, 28, 1724–1736. [Google Scholar] [CrossRef]
- Arjanggi, R.D.; Kansedo, J. Recent Advancement and Prospective of Waste Plastics as Biodiesel Additives: A Review. J. Energy Inst. 2020, 93, 934–952. [Google Scholar] [CrossRef]
- Gutierrez-Velasquez, E.I.; Monteiro, S.N.; Colorado, H.A. Characterization of Expanded Polystyrene Waste as Binder and Coating Material. Case Stud. Constr. Mater. 2022, 16, e00804. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Bolzan, A.; Machado, R.A.F. A Closed-Loop Process Design for Recycling Expanded Polystyrene Waste by Dissolution and Polymerization. Polymer 2020, 209, 122940. [Google Scholar] [CrossRef]
- Ayeleru, O.O.; Dlova, S.; Akinribide, O.J.; Olorundare, O.F.; Akbarzadeh, R.; Kempaiah, D.M.; Hall, C.; Ntuli, F.; Kupolati, W.K.; Olubambi, P.A. Nanoindentation Studies and Characterization of Hybrid Nanocomposites Based on Solvothermal Process. Inorg. Chem. Commun. 2020, 113, 107704. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, A.; Rabnawaz, M. Efficient Depolymerization of Polystyrene with Table Salt and Oxidized Copper. ACS Sustain. Chem. Eng. 2022, 10, 6493–6502. [Google Scholar] [CrossRef]
- Balema, V.P.; Hlova, I.Z.; Carnahan, S.L.; Seyedi, M.; Dolotko, O.; Rossini, A.J.; Luzinov, I. Depolymerization of Polystyrene under Ambient Conditions. New J. Chem. 2021, 45, 2935–2938. [Google Scholar] [CrossRef]
- Valášková, M.; Madejová, J.; Inayat, A.; Matějová, L.; Ritz, M.; Martaus, A.; Leštinský, P. Vermiculites from Brazil and Palabora: Structural Changes upon Heat Treatment and Influence on the Depolymerization of Polystyrene. Appl. Clay Sci. 2020, 192, 105639. [Google Scholar] [CrossRef]
- Achilias, D.S. Polymer Degradation Under Microwave Irradiation; Springer International: Cham, Switzerland, 2014; pp. 309–346. [Google Scholar]
- Bartoli, M.; Rosi, L.; Frediani, M.; Undri, A.; Frediani, P. Depolymerization of Polystyrene at Reduced Pressure through a Microwave Assisted Pyrolysis. J. Anal. Appl. Pyrolysis 2015, 113, 281–287. [Google Scholar] [CrossRef]
- Saha, N.; Banivaheb, S.; Toufiq Reza, M. Towards Solvothermal Upcycling of Mixed Plastic Wastes: Depolymerization Pathways of Waste Plastics in Sub- and Supercritical Toluene. Energy Convers. Manag. X 2022, 13, 100158. [Google Scholar] [CrossRef]
- Hu, K.; Tian, W.; Yang, Y.; Nie, G.; Zhou, P.; Wang, Y.; Duan, X.; Wang, S. Microplastics Remediation in Aqueous Systems: Strategies and Technologies. Water Res. 2021, 198, 117144. [Google Scholar] [CrossRef] [PubMed]
- Karthick, K.; Mohan Ramana, V.; Muralikrishnan, M.S.; Vishnuvardhan, N.; Naveen Kumar, S. Effects of Various Reinforcement on Mechanical Properties of Plastic Block: A Review. J. Phys. Conf. Ser. 2021, 2054, 012075. [Google Scholar] [CrossRef]
- Tittarelli, F.; Giosuè, C.; Mobili, A.; di Perna, C.; Monosi, S. Effect of Using Recycled Instead of Virgin EPS in Lightweight Mortars. Procedia Eng. 2016, 161, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Petrella, A.; Di Mundo, R.; Notarnicola, M. Recycled Expanded Polystyrene as Lightweight Aggregate for Environmentally Sustainable Cement Conglomerates. Materials 2020, 13, 988. [Google Scholar] [CrossRef] [Green Version]
- Rosca, B. Comparative Aspects Regarding a Novel Lightweight Concrete of Structural Grade Containing Brick Aggregate as Coarse Particles and Expanded Polystyrene Beads. Mater. Today Proc. 2021, 45, 4979–4986. [Google Scholar] [CrossRef]
- Koksal, F.; Mutluay, E.; Gencel, O. Characteristics of Isolation Mortars Produced with Expanded Vermiculite and Waste Expanded Polystyrene. Constr. Build. Mater. 2020, 236, 117789. [Google Scholar] [CrossRef]
- Nwaubani, S.O.; Parsons, L.A. Properties, Durability and Microstructure of Concrete Incorporating Waste Electrical and Electronic Plastics as Partial Replacement for Aggregates in Concrete. Case Stud. Constr. Mater. 2021, 15, e00731. [Google Scholar] [CrossRef]
- Hussein, W.A. Production of Lightweight Concrete by Using Polystyrene (Cork) Waste. J. Phys. Conf. Ser. 2021, 1973, 012128. [Google Scholar] [CrossRef]
- Reynoso, L.E.; Carrizo Romero, Á.B.; Viegas, G.M.; San Juan, G.A. Characterization of an Alternative Thermal Insulation Material Using Recycled Expanded Polystyrene. Constr. Build. Mater. 2021, 301, 124058. [Google Scholar] [CrossRef]
- Eskander, S.B.; Saleh, H.M.; Tawfik, M.E.; Bayoumi, T.A. Towards Potential Applications of Cement-Polymer Composites Based on Recycled Polystyrene Foam Wastes on Construction Fields: Impact of Exposure to Water Ecologies. Case Stud. Constr. Mater. 2021, 15, e00664. [Google Scholar] [CrossRef]
- Hamah Sor, N.; Hilal, N.; Faraj, R.H.; Ahmed, H.U.; Sherwani, A.F.H. Experimental and Empirical Evaluation of Strength for Sustainable Lightweight Self-Compacting Concrete by Recycling High Volume of Industrial Waste Materials. Eur. J. Environ. Civ. Eng. 2021, 1–18. [Google Scholar] [CrossRef]
- Hilal, N.; Hamah Sor, N.; Faraj, R.H. Development of Eco-Efficient Lightweight Self-Compacting Concrete with High Volume of Recycled EPS Waste Materials. Environ. Sci. Pollut. Res. 2021, 28, 50028–50051. [Google Scholar] [CrossRef] [PubMed]
- Ulfat, W.; Mohyuddin, A.; Amjad, M.; Saeed, S.; Mujahid, B. Recycling of Buffing Dust Tanneries Waste to Prepare Structural Thermal Insulation Panels. Mater. Res. Express 2021, 8, 125303. [Google Scholar] [CrossRef]
- Barnat-Hunek, D.; Góra, J.; Widomski, M. Durability of Hydrophobic/Icephobic Coatings in Protection of Lightweight Concrete with Waste Aggregate. Materials 2020, 14, 101. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, Z.; Neacșu, V.A.; Zhao, S.; Chai, Y.; Zhang, H. Recycling Plastic Waste into Multifunctional Superhydrophobic Textiles. Nano Res. 2022, 1–5. [Google Scholar] [CrossRef]
- Foti, D.; Voulgaridou, E.E.; Karastergiou, S.; Taghiyari, H.R.; Papadopoulos, A.N. Physical and Mechanical Properties of Eco-Friendly Composites Made from Wood Dust and Recycled Polystyrene. J. Renew. Mater. 2022, 10, 75–88. [Google Scholar] [CrossRef]
- Amadji, T.A.; Adjovi, E.C.; Gérard, J.; Barés, J.; Huon, V. Étude Des Propriétés Technologiques d’un Composite Bois-Plastique Élaboré Au Bénin. Bois Forets Trop. 2021, 348, 49–63. [Google Scholar] [CrossRef]
- Cebrian, A.V.S.; Carvalho, R.S.; Barreto, A.R.J.; Maturi, F.E.; Barud, H.S.; Silva, R.R.; Legnani, C.; Cremona, M.; Ribeiro, S.J.L. Development of Conformable Substrates for OLEDs Using Highly Transparent Bacterial Cellulose Modified with Recycled Polystyrene. Adv. Sustain. Syst. 2022, 6, 2000258. [Google Scholar] [CrossRef]
- Duarte Menezes, P.; De Meireles Carneiro, T.; Torres Marques, A. Composites for Life. U. Porto J. Eng. 2021, 7, 37–51. [Google Scholar] [CrossRef]
- Nikolaivits, E.; Pantelic, B.; Azeem, M.; Taxeidis, G.; Babu, R.; Topakas, E.; Brennan Fournet, M.; Nikodinovic-Runic, J. Progressing Plastics Circularity: A Review of Mechano-Biocatalytic Approaches for Waste Plastic (Re)Valorization. Front. Bioeng. Biotechnol. 2021, 9, 535. [Google Scholar] [CrossRef]
- Ballerstedt, H.; Tiso, T.; Wierckx, N.; Wei, R.; Averous, L.; Bornscheuer, U.; O’Connor, K.; Floehr, T.; Jupke, A.; Klankermayer, J.; et al. MIXed Plastics Biodegradation and UPcycling Using Microbial Communities: EU Horizon 2020 Project MIX-UP Started January 2020. Environ. Sci. Eur. 2021, 33, 99. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Kosewska, O.; Purwin, C.; Lipiński, K.; Ciesielski, S. Polyethylene, Polystyrene and Lignocellulose Wastes as Mealworm (Tenebrio molitor L.) Diets and Their Impact on the Breeding Condition, Biometric Parameters, Metabolism, and Digestive Microbiome. Sci. Total Environ. 2022, 832, 154758. [Google Scholar] [CrossRef]
- Machona, O.; Chidzwondo, F.; Mangoyi, R. Tenebrio Molitor: Possible Source of Polystyrene-Degrading Bacteria. BMC Biotechnol. 2022, 22, 2. [Google Scholar] [CrossRef]
- Matyja, K.; Rybak, J.; Hanus-Lorenz, B.; Wróbel, M.; Rutkowski, R. Effects of Polystyrene Diet on Tenebrio Molitor Larval Growth, Development and Survival: Dynamic Energy Budget (DEB) Model Analysis. Environ. Pollut. 2020, 264, 114740. [Google Scholar] [CrossRef]
- Peng, B.-Y.; Sun, Y.; Wu, Z.; Chen, J.; Shen, Z.; Zhou, X.; Wu, W.-M.; Zhang, Y. Biodegradation of Polystyrene and Low-Density Polyethylene by Zophobas Atratus Larvae: Fragmentation into Microplastics, Gut Microbiota Shift, and Microbial Functional Enzymes. J. Clean. Prod. 2022, 367, 132987. [Google Scholar] [CrossRef]
- Arunrattiyakorn, P.; Ponprateep, S.; Kaennonsang, N.; Charapok, Y.; Punphuet, Y.; Krajangsang, S.; Tangteerawatana, P.; Limtrakul, A. Biodegradation of Polystyrene by Three Bacterial Strains Isolated from the Gut of Superworms (Zophobas Atratus Larvae). J. Appl. Microbiol. 2022, 132, 2823–2831. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, L.; Li, X.; Wang, J.; Wang, H.; Chen, C.; Guo, H.; Han, T.; Zhou, A.; Zhao, X. Different Plastics Ingestion Preferences and Efficiencies of Superworm (Fab.) and Yellow Mealworm (Tenebrio Molitor Linn.) Associated with Distinct Gut Microbiome Changes. Sci. Total Environ. 2022, 837, 155719. [Google Scholar] [CrossRef]
- Ruiz Barrionuevo, J.M.; Martín, E.; Galindo Cardona, A.; Malizia, A.; Chalup, A.; de Cristóbal, R.E.; Monmany Garzia, A.C. Consumption of Low-Density Polyethylene, Polypropylene, and Polystyrene Materials by Larvae of the Greater Wax Moth, Galleria mellonella L. (Lepidoptera, Pyralidae), Impacts on Their Ontogeny. Environ. Sci. Pollut. Res. 2022, 29, 68132–68142. [Google Scholar] [CrossRef]
- Ruiz Barrionuevo, J.M.; Vilanova-Cuevas, B.; Alvarez, A.; Martín, E.; Malizia, A.; Galindo-Cardona, A.; de Cristóbal, R.E.; Occhionero, M.A.; Chalup, A.; Monmany-Garzia, A.C.; et al. The Bacterial and Fungal Gut Microbiota of the Greater Wax Moth, Galleria mellonella L. Consuming Polyethylene and Polystyrene. Front. Microbiol. 2022, 13, 918861. [Google Scholar] [CrossRef]
- Pinchi, J.E.; Galvez, J.O.; Castaneda-Olivera, C.A.; Alfaro, E.G.B. Environmental Biotechnology: Biodegradation of Microplastics with Larvae of Tenebrio Molitor and Galleria mellonella. Chem. Eng. Trans. 2022, 93, 187–192. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Huang, Z.; Zhou, N.; Xie, Y.; Tang, Y.; Hu, F.; Liu, G.; Zheng, H. Complete Digestion/Biodegradation of Polystyrene Microplastics by Greater Wax Moth (Galleria mellonella) Larvae: Direct in Vivo Evidence, Gut Microbiota Independence, and Potential Metabolic Pathways. J. Hazard. Mater. 2022, 423, 127213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Shen, Y.; Li, X.; Liu, X.; Qian, G.; Zhou, J. Feeding Preference of Insect Larvae to Waste Electrical and Electronic Equipment Plastics. Sci. Total Environ. 2022, 807, 151037. [Google Scholar] [CrossRef]
- Volk, R.; Stallkamp, C.; Steins, J.J.; Yogish, S.P.; Müller, R.C.; Stapf, D.; Schultmann, F. Techno-economic Assessment and Comparison of Different Plastic Recycling Pathways: A German Case Study. J. Ind. Ecol. 2021, 25, 1318–1337. [Google Scholar] [CrossRef]
- Marten, B.; Hicks, A. Expanded Polystyrene Life Cycle Analysis Literature Review: An Analysis for Different Disposal Scenarios. Sustain. J. Rec. 2018, 11, 29–35. [Google Scholar] [CrossRef]
- Ardolino, F.; Cardamone, G.F.; Arena, U. How to Enhance the Environmental Sustainability of WEEE Plastics Management: An LCA Study. Waste Manag. 2021, 135, 347–359. [Google Scholar] [CrossRef]
- da Silva Müller Teixeira, F.; de Carvalho Peres, A.C.; Gomes, T.S.; Visconte, L.L.Y.; Pacheco, E.B.A.V. A Review on the Applicability of Life Cycle Assessment to Evaluate the Technical and Environmental Properties of Waste Electrical and Electronic Equipment. J. Polym. Environ. 2021, 29, 1333–1349. [Google Scholar] [CrossRef]
- Hidalgo-Crespo, J.; Moreira, C.M.; Jervis, F.X.; Soto, M.; Amaya, J.L.; Banguera, L. Circular Economy of Expanded Polystyrene Container Production: Environmental Benefits of Household Waste Recycling Considering Renewable Energies. Energy Rep. 2022, 8, 306–311. [Google Scholar] [CrossRef]
- Ghosh, A. Performance Modifying Techniques for Recycled Thermoplastics. Resour. Conserv. Recycl. 2021, 175, 105887. [Google Scholar] [CrossRef]
- Zhi, X.; Liu, J.; Zhang, H.-B.; Hong, S.; Yu, Z.-Z. Simultaneous Enhancements in Electrical Conductivity and Toughness of Selectively Foamed Polycarbonate/Polystyrene/Carbon Nanotube Microcellular Foams. Compos. Part B Eng. 2018, 143, 161–167. [Google Scholar] [CrossRef]
- Martins, G.S.; Pereira, I.M.; Oréfice, R.L. Toughening Brittle Polymers with Shape Memory Polymers. Polymer 2018, 135, 30–38. [Google Scholar] [CrossRef]
- Ye, Z.; Yu, H.; Zheng, Z.; Hu, B.; Zhao, Y.; Wang, H. Janus Nanoshards Prepared Based on High Internal Phase Emulsion Templates for Compatibilizing Immiscible Polymer Blends. Macromolecules 2022, 55, 338–348. [Google Scholar] [CrossRef]
- Kim, D.K.; Hwang, S.S.; Yu, S. Poly(2,6-Dimethyl-1,4-Phenylene Ether)/Poly(Phenylene Sulfide)/Styrenic Block Copolymer Blends Compatibilized with Reactive Polystyrene. Mater. Chem. Phys. 2021, 273, 125100. [Google Scholar] [CrossRef]
- Biswal, M.; Mohanty, S.; Nayak, S.K. Compatibility Effect of R-ABS/r-HIPS/r-PS Blend Recovered from Waste Keyboard Plastics: Evaluation of Mechanical, Thermal and Morphological Performance. J. Polym. Res. 2021, 28, 129. [Google Scholar] [CrossRef]
- Grigorescu, R.M.; Ghioca, P.; Iancu, L.; Grigore, M.E.; Ion, R.; Nicolae, C.; Gabor, R.; Filipescu, M.I.; Rapa, M.; Trusca, R.D.; et al. Impact Strength Elastomer Composites Based on Polystyrene Components Separated from Waste Electrical and Electronic Equipment. J. Appl. Polym. Sci. 2020, 137, 48329. [Google Scholar] [CrossRef]
- Jaidev, K.; Suresh, S.S.; Gohatre, O.K.; Biswal, M.; Mohanty, S.; Nayak, S.K. Development of Recycled Blends Based on Cables and Wires with Plastic Cabinets: An Effective Solution for Value Addition of Hazardous Waste Plastics. Waste Manag. Res. 2020, 38, 312–321. [Google Scholar] [CrossRef]
- Hittini, W.; Mourad, A.-H.I.; Abu-Jdayil, B. Utilization of Devulcanized Waste Rubber Tire in Development of Heat Insulation Composite. J. Clean. Prod. 2021, 280, 124492. [Google Scholar] [CrossRef]
- Marín-Genescà, M.; García-Amorós, J.; Mujal-Rosas, R.; Vidal, L.M.; Arroyo, J.B.; Fajula, X.C. Ground Tire Rubber Recycling in Applications as Insulators in Polymeric Compounds, According to Spanish UNE Standards. Recycling 2020, 5, 16. [Google Scholar] [CrossRef]
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in Epoxy Resins. Polym. Rev. 2020, 60, 1–41. [Google Scholar] [CrossRef]
- Ding, Y.; Abeykoon, C.; Perera, Y.S. The Effects of Extrusion Parameters and Blend Composition on the Mechanical, Rheological and Thermal Properties of LDPE/PS/PMMA Ternary Polymer Blends. Adv. Ind. Manuf. Eng. 2022, 4, 100067. [Google Scholar] [CrossRef]
- Mendoza-Duarte, M.E.; Estrada-Moreno, I.A.; García-Casillas, P.E.; Vega-Rios, A. Stiff-Elongated Balance of PLA-Based Polymer Blends. Polymers 2021, 13, 4279. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhang, B.; Bates, F.S.; Lodge, T.P. Self-Assembly of Partially Charged Diblock Copolymer-Homopolymer Ternary Blends. Macromolecules 2022, 55, 4766–4775. [Google Scholar] [CrossRef]
- Pal, S.; Srivastava, R.K.; Nandan, B. Crystallization and Polymorphic Behaviour of Melt Miscible Blends of Crystalline Homopolymers with Close Melting Temperatures under Confinement. Polymer 2022, 256, 125249. [Google Scholar] [CrossRef]
- Ishigami, A.; Nishitsuji, S.; Kurose, T.; Ito, H. Evaluation of Toughness and Failure Mode of PA6/MSEBS/PS Ternary Blends with an Oil-Extended Viscoelastic Controlled Interface. Polymer 2019, 177, 57–64. [Google Scholar] [CrossRef]
- Jaidev, K.; Biswal, M.; Mohanty, S.; Nayak, S.K. Sustainable Waste Management of Engineering Plastics Generated from E-Waste: A Critical Evaluation of Mechanical, Thermal and Morphological Properties. J. Polym. Environ. 2021, 29, 1763–1776. [Google Scholar] [CrossRef]
- Cai, D.; Li, Y.; Wang, W.; Ma, Y.; Cao, N.; Zhang, J.; Pan, D.; Naik, N.; Wei, S.; Huang, M.; et al. Reinforcing and Toughening Blends of Recycled Acrylonitrile-Butadiene-Styrene/Recycled High-Impact Polystyrene through Ionic Crosslinking. Surf. Interfaces 2022, 28, 101607. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, J.; Li, P.; Ding, D.; Zheng, X.; Hu, C.; Gao, Z.; Hu, T.; Gong, X.; Wu, C. Melt Blending Modification of Commercial Polystyrene with Its Half Critical Molecular Weight, High Ion Content Ionomer, Poly(Styrene–Ran–Cinnamic Acid) Zn Salt, toward Heat Resistance Improvement. Polymers 2020, 12, 584. [Google Scholar] [CrossRef] [Green Version]
- Hayeemasae, N.; Ismail, H. Potential of Calcium Carbonate as Secondary Filler in Eggshell Powder Filled Recycled Polystyrene Composites. Polímeros 2021, 31. [Google Scholar] [CrossRef]
- Ferreira, E.H.C.; Lima, L.P.; Fechine, G.J.M. The “Superlubricity State” of Carbonaceous Fillers on Polymer Composites. Macromol. Chem. Phys. 2020, 221, 2000192. [Google Scholar] [CrossRef]
- Ab Ghani, N.F.; Mat Desa, M.S.Z.; Yusop, M.; Bijarimi, M.; Ramli, A.; Ali, M.F. Mechanical Properties of Styrene Butadiene Rubber Toughened Graphene Reinforced Polystyrene. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 052010. [Google Scholar] [CrossRef]
- Kong, J.; Tan, B.H.; Lu, X.; Li, Z.; He, C. Hybrid >POSSNanocomposites. In Silicon Containing Hybrid Copolymers; Wiley: Weinheim, Germany, 2020; pp. 201–237. [Google Scholar]
- Giovino, M.; Pribyl, J.; Benicewicz, B.; Bucinell, R.; Schadler, L. Mechanical Properties of Polymer Grafted Nanoparticle Composites. Nanocomposites 2018, 4, 244–252. [Google Scholar] [CrossRef]












































| Species | Wavenumber (cm−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1400 | 1300 | 1120 | 1100 | 1030 | 1015 | 910 | 885 | 870 | 790 | 760 | 750 | 710 | 690 | 670 | |
| Talcum | peak at 1010 | 761 | 670 | ||||||||||||
| Kaolin | 1114 | 1032 | 1008 | 914 | 791 | 754 | 696 | ||||||||
| CaCO3 | peak at 1406 | 873 | 712 | ||||||||||||
| TiO2 | 877 | peak at 650, shoulder at 570–540 | |||||||||||||
| CO2 | 670 | ||||||||||||||
| H2O | Noise-like | ||||||||||||||
| Parameters | Type Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |
| Does ignition occur? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Do flames reach 15 cm? | No | No | Yes | Yes | No | No | No | No | No |
| Time to reach 15 cm | - | - | 5 s | 2 s | - | - | - | - | - |
| Is the filter paper igniting? | No | No | No | No | No | No | No | No | No |
| Properties | Pure PS | Sample Composite |
|---|---|---|
| Density (Kg m−3) | 600 | 300 |
| Compression Strength (load in tons) | 5.2 | 6.25 |
| Water Absorption (%) | 5 | 7.5 |
| Thermal Conductivity (Wm−1 K−1) | 0.033 | 0.029 |
| Material | PB (μm2) | SAN (μm2) | PS (μm2) |
|---|---|---|---|
| HrAv-C1 | 0.14 | 5.2 | - |
| HrAv-C2 | 0.15 | 3.6 | - |
| HrAr-C1 | 0.37 | 11.33 | - |
| HrAr-C2 | 0.34 | 22.00 | - |
| ArHv-C1 | 0.21 | - | 4.2 |
| ArHv-C2 | 0.13 | - | 4.0 |
| ArHr-C1 | 0.11 | - | 3.7 |
| ArHr-C2 | 0.11 | - | 7.2 |
| Components Analyzed | Electrical Criterion | Mechanical Criterion | Application Standard | Polymers + GTR Suitable |
|---|---|---|---|---|
|
Insulation for electric shepherds |
Conductivity: <10−12 S cm−1 Tg δ < 104 |
Tensile strength: 12.5 MPa Elongation at break: 300% |
ITC-BT-39, 22, 23 24 UNE-EN 60335-2-76 IEC 60335-2-76 | Eva + 10% |
| Spacer for power lines |
Resistivity: >5.5 × 105 Ω∙cm |
Minimum tensile strength: 17.2 MPa Minimum elongation at break: 300% | IEC 61854 | EVA + 20% |
| Universal electrical cable joint |
Resistivity: >1012 Ω∙cm |
Tensile strength: 12.5 MPa Elongation at break: 400% | IEC 60840 UNE HD 628 | EVA + 10% |
| Filler for electrical applications |
Resistivity: >1012 Ω∙cm |
Tensile strength: 12.5 MPa Elongation at break: 350% |
UNE 53 602; UNE 53 510; UNE-HD 632; UNE-EN 60811-4-1 | EVA + 10% |
| Trays and pipes for electrical cables |
Resistivity: >1012 Ω∙cm |
Elongation at break: 80 ± 10% Tensile strength: 15 MPa |
UNE EN 61537 UNE EN 50085-1: IEC 61537 (EN 61537) |
PP + 10% EVA + 10% |
|
Footwear for work use (insulating) Insulating: High electrical resistance |
Resistivity: >106 Ω∙cm >109 Ω∙cm |
Tensile strength: 10–12 MPa Elongation at break: >450% |
UNE-EN ISO 20345/6/7:2005 UNE 53510 | EVA + 10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capricho, J.C.; Prasad, K.; Hameed, N.; Nikzad, M.; Salim, N. Upcycling Polystyrene. Polymers 2022, 14, 5010. https://doi.org/10.3390/polym14225010
Capricho JC, Prasad K, Hameed N, Nikzad M, Salim N. Upcycling Polystyrene. Polymers. 2022; 14(22):5010. https://doi.org/10.3390/polym14225010
Chicago/Turabian StyleCapricho, Jaworski C., Krishnamurthy Prasad, Nishar Hameed, Mostafa Nikzad, and Nisa Salim. 2022. "Upcycling Polystyrene" Polymers 14, no. 22: 5010. https://doi.org/10.3390/polym14225010