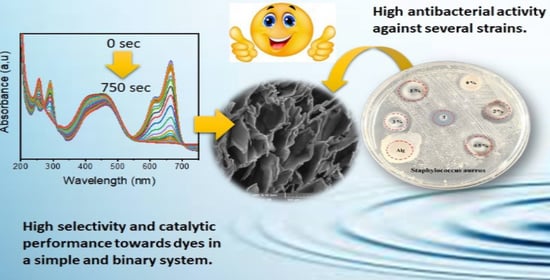
Catalytic Reduction of Dyes and Antibacterial Activity of AgNPs@Zn@Alginate Composite Aerogel Beads
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Preparation of Zn–Alginate(AgNPs)
2.3. Catalytic Test
2.4. Evaluation of Antibacterial Activity
2.5. Characterization of Composite Beads
3. Results and Discussion
3.1. Characterization of Materials
3.1.1. XRD
3.1.2. FTIR
3.1.3. TGA
3.1.4. SEM
3.1.5. EDS
3.2. Reduction of Dyes
3.2.1. Reduction of Organic Pollutants in a Simple System
- (a)
- Effect of the catalyst mass
- (b)
- Effect of silver content
- (c)
- Effect of the nature of the dyes
3.2.2. Reduction of Organic Pollutants in a Binary System
3.2.3. Catalyst Reuse
3.2.4. Mechanism of Reduction
3.3. Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teo, S.H.; Ng, C.H.; Islam, A.; Abdulkareem-Alsultan, G.; Joseph, C.G.; Janaun, J.; Taufiq-Yap, Y.H.; Khandaker, S.; Islam, G.J.; Znad, H.; et al. Sustainable toxic dyes removal with advanced materials for clean water production: A comprehensive review. J. Clean. Prod. 2022, 332, 130039. [Google Scholar] [CrossRef]
- Behera, M.; Nayak, J.; Banerjee, S.; Chakrabortty, S.; Tripathy, S.K. A review on the treatment of textile industry waste effluents towards the development of efficient mitigation strategy: An integrated system design approach. J. Environ. Chem. Eng. 2021, 9, 105277. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, H.; Alam, S. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Mekki, A.; Mokhtar, A.; Hachemaoui, M.; Beldjilali, M.; Meliani, M.F.; Zahmani, H.H.; Hacini, S.; Boukoussa, B. Fe and Ni nanoparticles-loaded zeolites as effective catalysts for catalytic reduction of organic pollutants. Microporous Mesoporous Mater. 2021, 310, 110597. [Google Scholar] [CrossRef]
- Mekki, A.; Hachemaoui, M.; Mokhtar, A.; Issam, I.; Bennabi, F.; Iqbal, J.; Rahmani, K.; Bengueddach, A.; Boukoussa, B. Catalytic behavior and antibacterial/antifungal activities of new MNPs/zeolite@alginate composite beads. Int. J. Biol. Macromol. 2022, 198, 37–45. [Google Scholar] [CrossRef]
- Asli, B.; Abdelkrim, S.; Zahraoui, M.; Mokhtar, A.; Hachemaoui, M.; Bennabi, F.; Ahmed, A.B.; Sardi, A.; Boukoussa, B. Catalytic Reduction and Antibacterial Activity of MCM-41 Modified by Silver Nanoparticles. Silicon 2022, 1–12. [Google Scholar] [CrossRef]
- Zaoui, F.; Sebba, F.Z.; Liras, M.; Sebti, H.; Hachemaoui, M.; Mokhtar, A.; Beldjilali, M.; Bounaceur, B.; Boukoussa, B. Ultrasonic preparation of a new composite poly(GMA)@Ru/TiO2@Fe3O4: Application in the catalytic reduction of organic pollutants. Mater. Chem. Phys. 2021, 260, 124146. [Google Scholar] [CrossRef]
- Xu, P.; Cen, C.; Zheng, M.; Wang, Y.; Wu, Z.; Teng, Z. A facile electrostatic droplets assisted synthesis of copper nanoparticles embedded magnetic carbon microspheres for highly effective catalytic reduction of 4-nitrophenol and Rhodamine B. Mater. Chem. Phys. 2020, 253, 123444. [Google Scholar] [CrossRef]
- Sun, H.; Abdeta, A.B.; Kuo, D.-H.; Wu, Q.; Guo, Y.; Zelekew, O.A.; Yuan, Z.; Lin, J.; Chen, X. Activated carbon supported CuSnOS catalyst with an efficient catalytic reduction of pollutants under dark condition. J. Mol. Liq. 2021, 334, 116079. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Z.; Fu, L.; Zhang, Y.; Yang, H.; Ouyang, J.; Chen, D. Silver nanoparticles assembled on modified sepiolite nanofibers for enhanced catalytic reduction of 4-nitrophenol. Appl. Clay Sci. 2018, 166, 166–173. [Google Scholar] [CrossRef]
- Zhang, N.; Peng, S.; Liu, Z.; Li, Y.; Huang, J.; Li, J.; Wan, H.; Zhou, S.; Gao, Z.; Chen, T. Ag NPs decorated on the magnetic Fe3O4@PDA as efficient catalyst for organic pollutants removal and as effective antimicrobial agent for microbial inhibition. J. Alloys Compd. 2022, 928, 167257. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Mokhtar, A.; Ismail, I.; Mohamedi, M.W.; Iqbal, J.; Taha, I.; Bennabi, F.; Zaoui, F.; Bengueddach, A.; Hamacha, R.; et al. M (M: Cu, Co, Cr or Fe) nanoparticles-loaded metal-organic framework MIL-101(Cr) material by sonication process: Catalytic activity and antibacterial properties. Microporous Mesoporous Mater. 2021, 323, 111244. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Sivamani, S.; Sivakumar, N.; Maran, J.P.; Hosseini-Bandegharaei, A. Ecofriendly biopolymers and composites: Preparation and their applications in water-treatment. Biotechnol. Adv. 2021, 52, 107815. [Google Scholar] [CrossRef]
- Khalil, H.A.; Yahya, E.B.; Jummaat, F.; Adnan, A.; Olaiya, N.; Rizal, S.; Abdullah, C.; Pasquini, D.; Thomas, S. Biopolymers based Aerogels: A Review on Revolutionary Solutions for Smart Therapeutics Delivery. Prog. Mater. Sci. 2023, 131, 101014. [Google Scholar] [CrossRef]
- Xu, T.; Liu, K.; Sheng, N.; Zhang, M.; Liu, W.; Liu, H.; Dai, L.; Zhang, X.; Si, C.; Du, H.; et al. Biopolymer-based hydrogel electrolytes for advanced energy storage/conversion devices: Properties, applications, and perspectives. Energy Storage Mater. 2022, 48, 244–262. [Google Scholar] [CrossRef]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Gopinath, K.P.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Qamar, S.A.; Qamar, M.; Basharat, A.; Bilal, M.; Cheng, H.; Iqbal, H.M. Alginate-based nano-adsorbent materials – Bioinspired solution to mitigate hazardous environmental pollutants. Chemosphere 2022, 288, 132618. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Al-Gethami, W.; Alhashmialameer, D.; Al-Qasmi, N.; Ismail, S.H.; Sadek, A.H. Design of a Novel Nanosensors Based on Green Synthesized CoFe2O4/Ca-Alginate Nanocomposite-Coated QCM for Rapid Detection of Pb(II) Ions. Nanomaterials 2022, 12, 3620. [Google Scholar] [CrossRef] [PubMed]
- Anwar, Y.; Ali, H.S.H.M.; Rehman, W.U.; Hemeg, H.A.; Khan, S.A. Antibacterial Films of Alginate-CoNi-Coated Cellulose Paper Stabilized Co NPs for Dyes and Nitrophenol Degradation. Polymers 2021, 13, 4122. [Google Scholar] [CrossRef]
- Filote, C.; Lanez, E.; Popa, V.I.; Lanez, T.; Volf, I. Characterization and Bioactivity of Polysaccharides Separated through a (Sequential) Biorefinery Process from Fucus spiralis Brown Macroalgae. Polymers 2022, 14, 4106. [Google Scholar] [CrossRef]
- Khan, S.B.; Bakhsh, E.M.; Akhtar, K.; Kamal, T.; Shen, Y.; Asiri, A.M. Copper Oxide-Antimony Oxide Entrapped Alginate Hydrogel as Efficient Catalyst for Selective Reduction of 2-Nitrophenol. Polymers 2022, 14, 458. [Google Scholar] [CrossRef]
- Kumar, R.; Oves, M.; Ansari, M.O.; Taleb, A.; Baraka, M.A.E.-F.; Alghamdi, M.A.; Al Makishah, N.H. Biopolymeric Ni3S4/Ag2S/TiO2/Calcium Alginate Aerogel for the Decontamination of Pharmaceutical Drug and Microbial Pollutants from Wastewater. Nanomaterials 2022, 12, 3642. [Google Scholar] [CrossRef]
- Sabater i Serra, R.; Molina-Mateo, J.; Torregrosa-Cabanilles, C.; Andrio-Balado, A.; Dueñas, J.M.M.; Serrano-Aroca, Á. Bio-Nanocomposite Hydrogel Based on Zinc Alginate/Graphene Oxide: Morphology, Structural Conformation, Thermal Behavior/Degradation, and Dielectric Properties. Polymers 2020, 12, 702. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Xia, K.; Fang, C.; Chen, X.; Ye, Y. Rapid Removal of Azophloxine via Catalytic Degradation by a Novel Heterogeneous Catalyst under Visible Light. Catalysts 2020, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhao, W.; Ning, F.; Zhen, J.; Qiang, H.; Zhang, Y.; Liu, F.; Jia, Z. Alginate Fiber-Enhanced Poly(vinyl alcohol) Hydrogels with Superior Lubricating Property and Biocompatibility. Polymers 2022, 14, 4063. [Google Scholar] [CrossRef]
- Ullah, K.; Khan, S.; Khan, M.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Mahmood, A.; Hussain, S.; Khan, S.B.; Khan, S.A. A bioresource catalyst system of alginate-starch-activated carbon microsphere templated Cu nanoparticles: Potentials in nitroarenes hydrogenation and dyes discoloration. Int. J. Biol. Macromol. 2022, 222, 887–901. [Google Scholar] [CrossRef]
- Shah, S.A.; Ahmad, Z.; Khan, S.A.; Al-Ghamdi, Y.O.; Bakhsh, E.M.; Khan, N.; Rehman, M.U.; Jabli, M.; Khan, S.B. Biomass impregnated zero-valent Ag and Cu supported-catalyst: Evaluation in the reduction of nitrophenol and discoloration of dyes in aqueous medium. J. Organomet. Chem. 2021, 938, 121756. [Google Scholar] [CrossRef]
- Riaz, M.; Khan, N.; Khan, S.A.; Saeeduddin; Ahmad, Z.; Khan, M.A.; Iqbal, M.; Hemeg, H.A.; Bakhsh, E.M.; Khan, S.B. Enhanced catalytic reduction/degradation of organic pollutants and antimicrobial activity with metallic nanoparticles immobilized on copolymer modified with NaY zeolite films. J. Mol. Liq. 2022, 359, 119246. [Google Scholar] [CrossRef]
- Khan, S.B.; Khan, M.S.J.; Bakhsh, E.M.; Akhtar, K.; Asiri, A.M. Metallic nanoparticles decorated chitosan hydrogel wrapped pencil graphite: Effective catalyst for reduction of water pollutants and hydrogen production. Surf. Interfaces 2022, 31, 102004. [Google Scholar] [CrossRef]
- Khan, S.B.; Ahmad, S.; Kamal, T.; Asiri, A.M.; Bakhsh, E.M. Metal nanoparticles decorated sodium alginate-carbon nitride composite beads as effective catalyst for the reduction of organic pollutants. Int. J. Biol. Macromol. 2020, 164, 1087–1098. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Mokhtar, A.; Mekki, A.; Zaoui, F.; Abdelkrim, S.; Hacini, S.; Boukoussa, B. Composites beads based on Fe3O4@MCM-41 and calcium alginate for enhanced catalytic reduction of organic dyes. Int. J. Biol. Macromol. 2020, 164, 468–479. [Google Scholar] [CrossRef]
- Ahmad, S.; Asiri, A.M.; Kamal, T.; Khan, S.B. Efficient reduction of organic pollutants and H2 generation using bimetallic nanoparticles coated alginate hydrogel beads. Microporous Mesoporous Mater. 2022, 341, 112065. [Google Scholar] [CrossRef]
- Khan, S.B.; Akhtar, K.; Bakhsh, E.M.; Kamal, T.; Asiri, A.M. Alginate biopolymer as a reactor container for copper oxide-tin oxide: Efficient nanocatalyst for reduction of different pollutants. Chemosphere 2022, 291, 132811. [Google Scholar] [CrossRef]
- Khan, S.A.; Mohammed, S.A.; Bakhsh, E.M.; Al-Ghamdi, Y.O.; Rauf, A.; Akhtar, K.; Begum, A.; Khan, S.B. Reduction of nitrophenol isomers and degradation of azo dyes through zero-valent Ni nanoparticles anchored on cellulose acetate coated Ce/Zr composite. J. Water Process Eng. 2021, 44, 102383. [Google Scholar] [CrossRef]
- Benali, F.; Boukoussa, B.; Ismail, I.; Hachemaoui, M.; Iqbal, J.; Taha, I.; Cherifi, Z.; Mokhtar, A. One pot preparation of CeO2@Alginate composite beads for the catalytic reduction of MB dye: Effect of cerium percentage. Surf. Interfaces 2021, 26, 101306. [Google Scholar] [CrossRef]
- Mokhtar, A.; Abdelkrim, S.; Zaoui, F.; Sassi, M.; Boukoussa, B. Improved Stability of Starch@Layered-Materials Composite Films for Methylene Blue Dye Adsorption in Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3826–3831. [Google Scholar] [CrossRef]
- Tafjord, J.; Rytter, E.; Holmen, A.; Myrstad, R.; Svenum, I.-H.; Christensen, B.E.; Yang, J. Transition-Metal Nanoparticle Catalysts Anchored on Carbon Supports via Short-Chain Alginate Linkers. ACS Appl. Nano Mater. 2021, 4, 3900–3910. [Google Scholar] [CrossRef]
- Amirnejat, S.; Nosrati, A.; Javanshir, S.; Naimi-Jamal, M.R. Superparamagnetic alginate-based nanocomposite modified by L-arginine: An eco-friendly bifunctional catalysts and an efficient antibacterial agent. Int. J. Biol. Macromol. 2020, 152, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, F. Green Approach for Metal-free One-pot Synthesis of Tetrahydrobenzo[b]pyrans with Sodium Alginate as a Reusable Bifunctional Biopolymeric Catalyst. Org. Prep. Proced. Int. 2022, 54, 306–311. [Google Scholar] [CrossRef]
- Simelane, N.P.; Asante, J.K.; Ndibewu, P.P.; Mramba, A.S.; Sibali, L.L. Biopolymer Composites For Removal of Toxic Organic Compounds In Pharmaceutical Effluents–A Review. Carbohydr. Polym. Technol. Appl. 2022, 4, 100239. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; El-Monaem, E.M.A.; Elshishini, H.M.; El-Aqapa, H.G.; Hosny, M.; Abdelfatah, A.M.; Ahmed, M.S.; Hammad, E.N.; El-Subruiti, G.M.; Fawzy, M.; et al. Recent developments in alginate-based adsorbents for removing phosphate ions from wastewater: A review. RSC Adv. 2022, 12, 8228–8248. [Google Scholar] [CrossRef]
- Chia, J.J.; Shameli, K.; Yusefi, M.; Ali, R.R.; Balasundram, V.; Teow, S.-Y. Preparation and Application of Cross-linked Alginate Nanoparticles as Drug Carrier: A Review. J. Res. Nanosci. Nanotechnol. 2022, 5, 1–11. [Google Scholar] [CrossRef]
- Dai, Q.; Huang, X.; Jia, R.; Fang, Y.; Qin, Z. Development of antibacterial film based on alginate fiber, and peanut red skin extract for food packaging. J. Food Eng. 2022, 330, 111106. [Google Scholar] [CrossRef]
- Dananjaya, S.; Kumar, R.S.; Yang, M.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Synthesis, characterization of ZnO-chitosan nanocomposites and evaluation of its antifungal activity against pathogenic Candida albicans. Int. J. Biol. Macromol. 2018, 108, 1281–1288. [Google Scholar] [CrossRef]
- Dey, S.; Kumar, V.P. Supported and un-supported zinc and chromium oxide catalysts for lower temperature CO oxidation: A review. Environ. Chall. 2021, 3, 100061. [Google Scholar] [CrossRef]
- Ravichandran, A.; Rathika, R.; Kumaresavanji, M. Non-linear optical behaviour and antibacterial activity of semi-organic picolinic acid zinc sulphate single crystal. Mater. Today Proc. 2022, 68, 502–505. [Google Scholar] [CrossRef]
- Camacho-Jiménez, L.; Álvarez-Sánchez, A.R.; Mejía-Ruíz, C.H. Silver nanoparticles (AgNPs) as antimicrobials in marine shrimp farming: A review. Aquac. Rep. 2020, 18, 100512. [Google Scholar] [CrossRef]
- Ardakani, L.S.; Surendar, A.; Thangavelu, L.; Mandal, T. Silver nanoparticles (Ag NPs) as catalyst in chemical reactions. Synth. Commun. 2021, 51, 1516–1536. [Google Scholar] [CrossRef]
- Abdelkrim, S.; Mokhtar, A.; Djelad, A.; Hachemaoui, M.; Boukoussa, B.; Sassi, M. Insights into catalytic reduction of dyes catalyzed by nanocomposite beads Alginate@Fe3O4: Experimental and DFT study on the mechanism of reduction. Colloids Surf. Physicochem. Eng. Asp. 2022, 650, 129595. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Mokhtar, A.; Abdelkrim, S.; Ouargli-Saker, R.; Zaoui, F.; Hamacha, R.; Zahmani, H.H.; Hacini, S.; Bengueddach, A.; Boukoussa, B. Improved Catalytic Activity of Composite Beads Calcium Alginate@MIL-101@Fe3O4 Towards Reduction Toxic Organic Dyes. J. Polym. Environ. 2021, 29, 3813–3826. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Tech. Bull. Regist. Med. Technol. 1966, 36, 49–52. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Krupa, I. Alginate-Mediated Synthesis of Hetero-Shaped Silver Nanoparticles and Their Hydrogen Peroxide Sensing Ability. Molecules 2020, 25, 435. [Google Scholar] [CrossRef] [Green Version]
- Ramkumar, S.R.S.; Sivakumar, N.; Selvakumar, G.; Selvankumar, T.; Sudhakar, C.; Ashokkumar, B.; Karthi, S. Green synthesized silver nanoparticles from Garcinia imberti bourd and their impact on root canal pathogens and HepG2 cell lines. RSC Adv. 2017, 7, 34548–34555. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. In Handbook of Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Boukoussa, B.; Ismail, I.; Mokhtar, A.; Taha, I.; Iqbal, J.; Hacini, S.; Bengueddach, A.; Hamacha, R. CuNPs-loaded amines-functionalized-SBA-15 as effective catalysts for catalytic reduction of cationic and anionic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126729. [Google Scholar] [CrossRef]
- Benhadria, N.; Hachemaoui, M.; Zaoui, F.; Mokhtar, A.; Boukreris, S.; Attar, T.; Belarbi, L.; Boukoussa, B. Catalytic Reduction of Methylene Blue Dye by Copper Oxide Nanoparticles. J. Clust. Sci. 2022, 33, 249–260. [Google Scholar] [CrossRef]
- Benmaati, A.; Boukoussa, B.; Aoul, R.H.; Hachemaoui, M.; Kerbadou, R.M.; Zahmani, H.H.; Hacini, S. Insights into Catalytic Reduction of Organic Pollutants Catalyzed by Nanoparticles Supported on Zeolite Clinoptilolite. Silicon 2022, 14, 8831–8843. [Google Scholar] [CrossRef]
- Belkaid, N.; Boukoussa, B.; Mokhtar, A.; Hachemaoui, M.; Beldjilali, M.; Mekki, A.; Hacini, S.; Bengueddach, A.; Hamacha, R. Antibacterial Activity and Catalytic Reduction of 4-Nitrophenol and Methylene Blue on MCM-41 Modified by CuNPs. Silicon 2022, 14, 8505–8516. [Google Scholar] [CrossRef]
- Das, R.; Sypu, V.S.; Paumo, H.K.; Bhaumik, M.; Maharaj, V.; Maity, A. Silver decorated magnetic nanocomposite (Fe3O4@PPy-MAA/Ag) as highly active catalyst towards reduction of 4-nitrophenol and toxic organic dyes. Appl. Catal. B Environ. 2019, 244, 546–558. [Google Scholar] [CrossRef]
- Fu, Y.; Qin, L.; Huang, D.; Zeng, G.; Lai, C.; Li, B.; He, J.; Yi, H.; Zhang, M.; Cheng, M.; et al. Chitosan functionalized activated coke for Au nanoparticles anchoring: Green synthesis and catalytic activities in hydrogenation of nitrophenols and azo dyes. Appl. Catal. B Environ. 2019, 255, 117740. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Anand, K.K.H.; Mandal, B.K. Gold nanoparticles—Synthesis by Sterculia acuminata extract and its catalytic efficiency in alleviating different organic dyes. J. Mol. Liq. 2015, 211, 868–875. [Google Scholar] [CrossRef]
- Cui, X.; Zheng, Y.; Tian, M.; Dong, Z. Palladium nanoparticles supported on SiO2@Fe3O4@m-MnO2 mesoporous microspheres as a highly efficient and recyclable catalyst for hydrodechlorination of 2,4-dichlorophenol and reduction of nitroaromatic compounds and organic dyes. Mol. Catal. 2017, 433, 202–211. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Boukoussa, B.; Mokhtar, A.; Mekki, A.; Beldjilali, M.; Benaissa, M.; Zaoui, F.; Hakiki, A.; Chaibi, W.; Sassi, M.; et al. Dyes adsorption, antifungal and antibacterial properties of metal loaded mesoporous silica: Effect of metal and calcination treatment. Mater. Chem. Phys. 2020, 256, 123704. [Google Scholar] [CrossRef]
- Habila, I.; Saoudi, M.; Berrah, F.; Benmerad, B.; Boudraa, M.; Merazig, H.; Bouacida, S. A new complex of Zinc (II) with sulfamethoxazole ligand: Synthesis, crystal structure, Hirshfeld surface analysis, thermal properties, DFT calculations and antibacterial/antifungal activities. J. Mol. Struct. 2021, 1244, 130903. [Google Scholar] [CrossRef]
- Aslinjensipriya, A.; Reena, R.S.; Infantiya, S.G.; Ragu, R.; Das, S.J. Uncovering the replacement of Zn2+ ions on nano-structural, opto/magneto/electrical, antibacterial and antifungal attributes of nickel oxide nanoparticles via sol-gel strategy. J. Solid State Chem. 2022, 311, 123146. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, Q.; He, Y. Nano–Ag: Environmental applications and perspectives. Sci. Total Environ. 2022, 829, 154644. [Google Scholar] [CrossRef]












| Zn–AlG (Ag 0.5%) | Zn–AlG (Ag 1%) | Zn–AlG (Ag 2%) | Zn–AlG (Ag 3%) | Zn–AlG | Na–AlG | Gent | |
|---|---|---|---|---|---|---|---|
| E.c | 15 mm | 19 mm | 17 mm | 25 mm | 20 mm | 19 mm | 26 mm |
| P.a | 20 mm | 16 mm | 22 mm | 19 mm | 0 mm | 19 mm | 27 mm |
| S.a | 17 mm | 18 mm | 16 mm | 18 mm | 4 mm | 19 mm | 28 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benali, F.; Boukoussa, B.; Benkhedouda, N.-E.-H.; Cheddad, A.; Issam, I.; Iqbal, J.; Hachemaoui, M.; Abboud, M.; Mokhtar, A. Catalytic Reduction of Dyes and Antibacterial Activity of AgNPs@Zn@Alginate Composite Aerogel Beads. Polymers 2022, 14, 4829. https://doi.org/10.3390/polym14224829
Benali F, Boukoussa B, Benkhedouda N-E-H, Cheddad A, Issam I, Iqbal J, Hachemaoui M, Abboud M, Mokhtar A. Catalytic Reduction of Dyes and Antibacterial Activity of AgNPs@Zn@Alginate Composite Aerogel Beads. Polymers. 2022; 14(22):4829. https://doi.org/10.3390/polym14224829
Chicago/Turabian StyleBenali, Fadila, Bouhadjar Boukoussa, Nour-El-Houda Benkhedouda, Amina Cheddad, Ismail Issam, Jibran Iqbal, Mohammed Hachemaoui, Mohamed Abboud, and Adel Mokhtar. 2022. "Catalytic Reduction of Dyes and Antibacterial Activity of AgNPs@Zn@Alginate Composite Aerogel Beads" Polymers 14, no. 22: 4829. https://doi.org/10.3390/polym14224829