Plastic Waste Upcycling: A Sustainable Solution for Waste Management, Product Development, and Circular Economy
Abstract
:1. Introduction
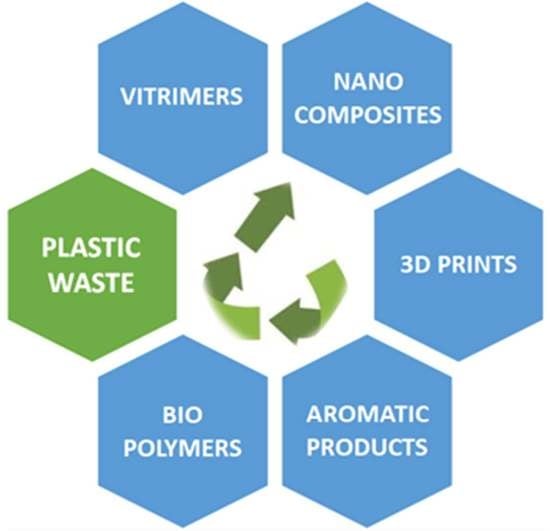
2. Upcycling of Plastic Waste
2.1. Vitrimerization
2.2. Nanocomposite Fabrication
2.3. Additive Manufacturing
2.4. Catalytic Transformation of Waste Plastic for the Production of Fine Chemicals and Carbon Materials
2.4.1. Nanocatalyzed Pyrolysis
2.4.2. Nanocatalyzed Gasification
2.4.3. Nanocatalyzed Hydrogenolysis and Hydrocracking
2.4.4. Nanocatalyzed Photoreforming
2.4.5. Nanocatalyzed Electroreforming
2.5. Industrial Biotechnology
2.5.1. Enzymatic Depolymerization
2.5.2. Biopolymer Synthesis
3. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millet, H.; Vangheluwe, P.; Block, C.; Sevenster, A.; Garcia, L.; Antonopoulos, R. The Nature of Plastics and Their Societal Usage. In Plastics and the Environment; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–20. [Google Scholar]
- Cabernard, L.; Pfister, S.; Oberschelp, C.; Hellweg, S. Growing environmental footprint of plastics driven by coal combustion. Nat. Sustain. 2021, 5, 139–148. [Google Scholar] [CrossRef]
- Zheng, J.; Suh, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Das, S.; Sharma, P.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Jawahir, I.; Bradley, R. Technological Elements of Circular Economy and the Principles of 6R-Based Closed-loop Material Flow in Sustainable Manufacturing. Procedia CIRP 2016, 40, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste–A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Okan, M.; Aydin, H.M.; Barsbay, M. Current approaches to waste polymer utilization and minimization: A review. J. Chem. Technol. Biotechnol. 2019, 94, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Albertsson, A.-C.; Hakkarainen, M. Designed to degrade. Science 2017, 358, 872–873. [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Anonymous. The future of plastic. Nat. Commun. 2018, 9, 2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Podgórski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward Stimuli-Responsive Dynamic Thermosets through Continuous Development and Improvements in Covalent Adaptable Networks (CANs). Adv. Mater. 2020, 32, e1906876. [Google Scholar] [CrossRef]
- Zheng, J.; Png, Z.M.; Ng, S.H.; Tham, G.X.; Ye, E.; Goh, S.S.; Loh, X.J.; Li, Z. Vitrimers: Current research trends and their emerging applications. Mater. Today 2021, 51, 586–625. [Google Scholar] [CrossRef]
- Hayashi, M. Implantation of Recyclability and Healability into Cross-Linked Commercial Polymers by Applying the Vitrimer Concept. Polymers 2020, 12, 1322. [Google Scholar] [CrossRef]
- Qiu, J.; Ma, S.; Wang, S.; Tang, Z.; Li, Q.; Tian, A.; Xu, X.; Wang, B.; Lu, N.; Zhu, J. Upcycling of Polyethylene Terephthalate to Continuously Reprocessable Vitrimers through Reactive Extrusion. Macromolecules 2021, 54, 703–712. [Google Scholar] [CrossRef]
- Caffy, F.; Nicolaÿ, R. Transformation of polyethylene into a vitrimer by nitroxide radical coupling of a bis-dioxaborolane. Polym. Chem. 2019, 10, 3107–3115. [Google Scholar] [CrossRef]
- Saed, M.O.; Lin, X.; Terentjev, E.M. Dynamic Semicrystalline Networks of Polypropylene with Thiol-Anhydride Exchangeable Crosslinks. ACS Appl. Mater. Interfaces 2021, 13, 42044–42051. [Google Scholar] [CrossRef]
- Kar, G.P.P.; Saed, M.O.; Terentjev, E.M. Scalable upcycling of thermoplastic polyolefins into vitrimers through transesterification. J. Mater. Chem. A 2020, 8, 24137–24147. [Google Scholar] [CrossRef]
- Hubbard, A.M.; Ren, Y.; Papaioannou, P.; Sarvestani, A.; Picu, C.R.; Konkolewicz, D.; Roy, A.K.; Varshney, V.; Nepal, D. Vitrimer Composites: Understanding the Role of Filler in Vitrimer Applicability. ACS Appl. Polym. Mater. 2022, 4, 6374–6385. [Google Scholar] [CrossRef]
- Alabiso, W.; Schlögl, S. The Impact of Vitrimers on the Industry of the Future: Chemistry, Properties and Sustainable Forward-Looking Applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Zeebaree, S.S.; Zeebaree, A.S.; Zebari, O.H.; Saeed, I. The Efficient Removal of Methylene Blue Dye Using CuO/PET Nanocomposite in Aqueous Solutions. Catalysts 2021, 11, 241. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Sharkawy, R.M.; Allam, E.A.; Elsaman, R.; El-Taher, A. Fabrication and characterization of phosphotungstic acid—Copper oxide nanoparticles—Plastic waste nanocomposites for enhanced radiation-shielding. J. Alloys Compd. 2019, 803, 768–777. [Google Scholar] [CrossRef]
- Fan, C.; Luo, Y. Polyethylene film waste-derived porous nanocomposites with superior mechanical robustness and excellent UV resistance as supported substrates for the development of multifunctional materials. J. Mater. Sci. 2020, 55, 10942–10952. [Google Scholar] [CrossRef]
- Yang, S.; Bai, S.; Duan, W.; Wang, Q. Production of Value-Added Composites from Aluminum–Plastic Package Waste via Solid-State Shear Milling Process. ACS Sustain. Chem. Eng. 2018, 6, 4282–4293. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.; Bai, S.; Wang, Q. Fabrication of Morphologically Controlled Composites with High Thermal Conductivity and Dielectric Performance from Aluminum Nanoflake and Recycled Plastic Package. ACS Appl. Mater. Interfaces 2019, 11, 3388–3399. [Google Scholar] [CrossRef]
- de Assis, G.C.; Skovroinski, E.; Leite, V.D.; Rodrigues, M.O.; Galembeck, A.; Alves, M.C.; Eastoe, J.; de Oliveira, R.J. Conversion of “Waste Plastic” into Photocatalytic Nanofoams for Environmental Remediation. ACS Appl. Mater. Interfaces 2018, 10, 8077–8085. [Google Scholar] [CrossRef] [Green Version]
- Uddin, N.; Desai, F.J.; Subeshan, B.; Rahman, M.M.; Asmatulu, E. Sustainable atmospheric fog water generator through superhydrophobic electrospun nanocomposite fibers of recycled expanded polystyrene foams. Surfaces Interfaces 2021, 25, 101169. [Google Scholar] [CrossRef]
- Mir, R.A.; Pandey, O. Waste plastic derived carbon supported Mo2C composite catalysts for hydrogen production and energy storage applications. J. Clean. Prod. 2019, 218, 644–655. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Haq, M.I.U. 3D printing—A review of processes, materials and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Fico, D.; Rizzo, D.; Casciaro, R.; Corcione, C.E. A Review of Polymer-Based Materials for Fused Filament Fabrication (FFF): Focus on Sustainability and Recycled Materials. Polymers 2022, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Rao, C.; Gu, F.; Sharmin, N.; Fu, J. Close-looped recycling of polylactic acid used in 3D printing: An experimental investigation and life cycle assessment. J. Clean. Prod. 2018, 197, 1046–1055. [Google Scholar] [CrossRef]
- Pavlo, S.; Fabio, C.; Hakim, B.; Mauricio, C. 3D-Printing Based Distributed Plastic Recycling: A Conceptual Model for Closed-Loop Supply Chain Design. In Proceedings of the 2018 IEEE International Conference on Engineering, Technology and Innovation (ICE/ITMC), Stuttgart, Germany, 17–20 June 2018; pp. 1–8. [Google Scholar]
- Vidakis, N.; Petousis, M.; Tzounis, L.; Maniadi, A.; Velidakis, E.; Mountakis, N.; Kechagias, J.D. Sustainable Additive Manufacturing: Mechanical Response of Polyamide 12 over Multiple Recycling Processes. Materials 2021, 14, 466. [Google Scholar] [CrossRef]
- Zander, N.E. Recycled Polymer Feedstocks for Material Extrusion Additive Manufacturing. In Polymer-Based Additive Manufacturing: Recent Developments; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2019; Volume 1315, pp. 37–51. [Google Scholar]
- Idrees, M.; Jeelani, S.; Rangari, V. Three-Dimensional-Printed Sustainable Biochar-Recycled PET Composites. ACS Sustain. Chem. Eng. 2018, 6, 13940–13948. [Google Scholar] [CrossRef]
- Carrete, I.A.; Quiñonez, P.A.; Bermudez, D.; Roberson, D.A. Incorporating Textile-Derived Cellulose Fibers for the Strengthening of Recycled Polyethylene Terephthalate for 3D Printing Feedstock Materials. J. Polym. Environ. 2021, 29, 662–671. [Google Scholar] [CrossRef]
- Bex, G.J.P.; Ingenhut, B.L.J.; Cate, T.T.; Sezen, M.; Ozkoc, G. Sustainable approach to produce 3D-printed continuous carbon fiber composites: “A comparison of virgin and recycled PETG”. Polym. Compos. 2021, 42, 4253–4264. [Google Scholar] [CrossRef]
- Mejia, E.B.; Al-Maqdi, S.; Alkaabi, M.; Alhammadi, A.; Alkaabi, M.; Cherupurakal, N.; Mourad, A.I. Upcycling of HDPE Waste using Additive Manufacturing: Feasibility and Challenges. In Proceedings of the 2020 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 4 February–9 April 2020; pp. 1–6. [Google Scholar]
- Borkar, A.; Hendlmeier, A.; Simon, Z.; Randall, J.D.; Stojcevski, F.; Henderson, L.C. A comparison of mechanical properties of recycled high-density polyethylene/waste carbon fiber via injection molding and 3D printing. Polym. Compos. 2022, 43, 2408–2418. [Google Scholar] [CrossRef]
- Zander, N.E.; Park, J.H.; Boelter, Z.R.; Gillan, M.A. Recycled Cellulose Polypropylene Composite Feedstocks for Material Extrusion Additive Manufacturing. ACS Omega 2019, 4, 13879–13888. [Google Scholar] [CrossRef] [Green Version]
- Stoof, D.; Pickering, K. Sustainable composite fused deposition modelling filament using recycled pre-consumer polypropylene. Compos. Part B Eng. 2018, 135, 110–118. [Google Scholar] [CrossRef]
- Morales, M.A.; Maranon, A.; Hernandez, C.; Porras, A. Development and Characterization of a 3D Printed Cocoa Bean Shell Filled Recycled Polypropylene for Sustainable Composites. Polymers 2021, 13, 3162. [Google Scholar] [CrossRef]
- Morales, M.; Martinez, C.A.; Maranon, A.; Hernandez, C.; Michaud, V.; Porras, A. Development and Characterization of Rice Husk and Recycled Polypropylene Composite Filaments for 3D Printing. Polymers 2021, 13, 1067. [Google Scholar] [CrossRef]
- Zander, N.E.; Gillan, M.; Burckhard, Z.; Gardea, F. Recycled polypropylene blends as novel 3D printing materials. Addit. Manuf. 2019, 25, 122–130. [Google Scholar] [CrossRef]
- Das, C.; Tamrakar, S.; Kiziltas, A.; Xie, X. Incorporation of Biochar to Improve Mechanical, Thermal and Electrical Properties of Polymer Composites. Polymers 2021, 13, 2663. [Google Scholar] [CrossRef] [PubMed]
- Gudadhe, A.A.; Bachhar, N.; Kumar, A.; Andrade, P.; Kumaraswamy, G. Three-Dimensional Printing with Waste High-Density Polyethylene. ACS Appl. Polym. Mater. 2019, 1, 3157–3164. [Google Scholar] [CrossRef]
- Amza, C.; Zapciu, A.; Constantin, G.; Baciu, F.; Vasile, M. Enhancing Mechanical Properties of Polymer 3D Printed Parts. Polymers 2021, 13, 562. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, K.; Zhang, X.; Yu, K.; Zhang, H.; He, J.; Ju, Y.; Liu, J. From plastic waste to wealth using chemical recycling: A review. J. Environ. Chem. Eng. 2022, 10, 106867. [Google Scholar] [CrossRef]
- Wang, C.; Han, H.; Wu, Y.; Astruc, D. Nanocatalyzed upcycling of the plastic wastes for a circular economy. Co-ord. Chem. Rev. 2022, 458, 214422. [Google Scholar] [CrossRef]
- Diaz-Silvarrey, L.S.; Zhang, K.; Phan, A.N. Monomer recovery through advanced pyrolysis of waste high density polyethylene (HDPE). Green Chem. 2018, 20, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
- Cai, N.; Li, X.; Xia, S.; Sun, L.; Hu, J.; Bartocci, P.; Fantozzi, F.; Williams, P.T.; Yang, H.; Chen, H. Pyrolysis-catalysis of different waste plastics over Fe/Al2O3 catalyst: High-value hydrogen, liquid fuels, carbon nanotubes and possible reaction mechanisms. Energy Convers. Manag. 2021, 229, 113794. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructure carbon materials via non-oxidative thermal degradation of real polypropylene waste plastic using La2O3 supported Ni and Ni–Cu catalysts. Polym. Degrad. Stab. 2019, 167, 157–169. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructured carbon materials using Fe–Mo/MgO catalysts via mild catalytic pyrolysis of polyethylene waste. Chem. Eng. J. 2018, 354, 802–816. [Google Scholar] [CrossRef]
- Al-Asadi, M.; Miskolczi, N.; Eller, Z. Pyrolysis-gasification of wastes plastics for syngas production using metal modified zeolite catalysts under different ratio of nitrogen/oxygen. J. Clean. Prod. 2020, 271, 122186. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Investigation of nickel-impregnated zeolite catalysts for hydrogen/syngas production from the catalytic reforming of waste polyethylene. Appl. Catal. B Environ. 2018, 227, 477–487. [Google Scholar] [CrossRef]
- Wu, S.-L.; Kuo, J.-H.; Wey, M.-Y. Design of catalysts comprising a nickel core and ceria shell for hydrogen production from plastic waste gasification: An integrated test for anti-coking and catalytic performance. Catal. Sci. Technol. 2020, 10, 3975–3984. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, S.; Zhang, H.; Liu, X.; Xiong, Y. High quality H2-rich syngas production from pyrolysis-gasification of biomass and plastic wastes by Ni–Fe@Nanofibers/Porous carbon catalyst. Int. J. Hydrogen Energy 2019, 44, 26193–26203. [Google Scholar] [CrossRef]
- Arregi, A.; Abbas-Abadi, M.S.; Lopez, G.; Santamaria, L.; Artetxe, M.; Bilbao, J.; Olazar, M. CeO2 and La2O3 Promoters in the Steam Reforming of Polyolefinic Waste Plastic Pyrolysis Volatiles on Ni-Based Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 17307–17321. [Google Scholar] [CrossRef]
- Utami, M.; Wijaya, K.; Trisunaryanti, W. Pt-promoted sulfated zirconia as catalyst for hydrocracking of LDPE plastic waste into liquid fuels. Mater. Chem. Phys. 2018, 213, 548–555. [Google Scholar] [CrossRef]
- Bin Jumah, A.; Anbumuthu, V.; Tedstone, A.A.; Garforth, A.A. Catalyzing the Hydrocracking of Low Density Polyethylene. Ind. Eng. Chem. Res. 2019, 58, 20601–20609. [Google Scholar] [CrossRef]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; LaPointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling Single-Use Polyethylene into High-Quality Liquid Products. ACS Central Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Tennakoon, A.; Wu, X.; Paterson, A.L.; Patnaik, S.; Pei, Y.; Lapointe, A.M.; Ammal, S.C.; Hackler, R.A.; Heyden, A.; Slowing, I.I.; et al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat. Catal. 2020, 3, 893–901. [Google Scholar] [CrossRef]
- Vance, B.C.; Kots, P.A.; Wang, C.; Hinton, Z.R.; Quinn, C.M.; Epps, T.H.; Korley, L.T.; Vlachos, D.G. Single pot catalyst strategy to branched products via adhesive isomerization and hydrocracking of polyethylene over platinum tungstated zirconia. Appl. Catal. B Environ. 2021, 299, 120483. [Google Scholar] [CrossRef]
- Liu, S.; Kots, P.A.; Vance, B.C.; Danielson, A.; Vlachos, D.G. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 2021, 7, eabf8283. [Google Scholar] [CrossRef] [PubMed]
- Rorrer, J.E.; Beckham, G.T.; Román-Leshkov, Y. Conversion of Polyolefin Waste to Liquid Alkanes with Ru-Based Catalysts under Mild Conditions. JACS Au 2021, 1, 8–12. [Google Scholar] [CrossRef]
- Jia, C.; Xie, S.; Zhang, W.; Intan, N.N.; Sampath, J.; Pfaendtner, J.; Lin, H. Deconstruction of high-density polyethylene into liquid hydrocarbon fuels and lubricants by hydrogenolysis over Ru catalyst. Chem Catal. 2021, 1, 437–455. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Troyano-Valls, C.; Beckham, G.T.; Román-Leshkov, Y. Hydrogenolysis of Polypropylene and Mixed Polyolefin Plastic Waste over Ru/C to Produce Liquid Alkanes. ACS Sustain. Chem. Eng. 2021, 9, 11661–11666. [Google Scholar] [CrossRef]
- Nakaji, Y.; Tamura, M.; Miyaoka, S.; Kumagai, S.; Tanji, M.; Nakagawa, Y.; Yoshioka, T.; Tomishige, K. Low-temperature catalytic upgrading of waste polyolefinic plastics into liquid fuels and waxes. Appl. Catal. B Environ. 2021, 285, 119805. [Google Scholar] [CrossRef]
- Kots, P.A.; Liu, S.; Vance, B.C.; Wang, C.; Sheehan, J.D.; Vlachos, D.G. Polypropylene Plastic Waste Conversion to Lubricants over Ru/TiO2 Catalysts. ACS Catal. 2021, 11, 8104–8115. [Google Scholar] [CrossRef]
- Wang, C.; Xie, T.; Kots, P.A.; Vance, B.C.; Yu, K.; Kumar, P.; Fu, J.; Liu, S.; Tsilomelekis, G.; Stach, E.A.; et al. Polyethylene Hydrogenolysis at Mild Conditions over Ruthenium on Tungstated Zirconia. JACS Au 2021, 1, 1422–1434. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, Y.; Furukawa, S.; Xia, J.; Sun, C.; Hülsey, M.J.; Wang, H.; Guo, Y.; Liu, X.; Yan, N. Towards the Circular Economy: Converting Aromatic Plastic Waste Back to Arenes over a Ru/Nb2O5 Catalyst. Angew. Chem. Int. Ed. 2021, 60, 5527–5535. [Google Scholar] [CrossRef]
- Tamura, M.; Miyaoka, S.; Nakaji, Y.; Tanji, M.; Kumagai, S.; Nakagawa, Y.; Yoshioka, T.; Tomishige, K. Structure-activity relationship in hydrogenolysis of polyolefins over Ru/support catalysts. Appl. Catal. B Environ. 2022, 318, 121870. [Google Scholar] [CrossRef]
- Lee, W.-T.; Bobbink, F.D.; van Muyden, A.P.; Lin, K.-H.; Corminboeuf, C.; Zamani, R.R.; Dyson, P.J. Catalytic hydrocracking of synthetic polymers into grid-compatible gas streams. Cell Rep. Phys. Sci. 2021, 2, 100332. [Google Scholar] [CrossRef]
- Hongkailers, S.; Jing, Y.; Wang, Y.; Hinchiranan, N.; Yan, N. Recovery of Arenes from Polyethylene Terephthalate (PET) over a Co/TiO 2 Catalyst. ChemSusChem 2021, 14, 4330–4339. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ma, B.; Chen, S.; Tian, J.; Zhao, C. Converting waste PET plastics into automobile fuels and antifreeze components. Nat. Commun. 2022, 13, 3343. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, N.; Li, G.; Wang, A.; Cong, Y.; Xu, G.; Wang, X.; Zhang, T. Synthesis of gasoline and jet fuel range cycloalkanes and aromatics from poly(ethylene terephthalate) waste. Green Chem. 2019, 21, 2709–2719. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.-H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.-U.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete Photocatalytic Mineralization of Microplastic on TiO2 Nanoparticle Film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef] [Green Version]
- Uekert, T.; Kuehnel, M.F.; Wakerley, D.W.; Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 2018, 11, 2853–2857. [Google Scholar] [CrossRef] [Green Version]
- Uekert, T.; Kasap, H.; Reisner, E. Photoreforming of Nonrecyclable Plastic Waste over a Carbon Nitride/Nickel Phosphide Catalyst. J. Am. Chem. Soc. 2019, 141, 15201–15210. [Google Scholar] [CrossRef] [Green Version]
- Pichler, C.M.; Bhattacharjee, S.; Rahaman, M.; Uekert, T.; Reisner, E. Conversion of Polyethylene Waste into Gaseous Hydrocarbons via Integrated Tandem Chemical–Photo/Electrocatalytic Processes. ACS Catal. 2021, 11, 9159–9167. [Google Scholar] [CrossRef]
- Shi, R.; Liu, K.-S.; Liu, F.; Yang, X.; Hou, C.-C.; Chen, Y. Electrocatalytic reforming of waste plastics into high value-added chemicals and hydrogen fuel. Chem. Commun. 2021, 57, 12595–12598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ren, Y.; Li, Z.; Xu, M.; Wang, Y.; Ge, R.; Kong, X.; Zheng, L.; Duan, H. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel. Nat. Commun. 2021, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, C.; Lee, J. Reduction of polycyclic compounds and biphenyls generated by pyrolysis of industrial plastic waste by using supported metal catalysts: A case study of polyethylene terephthalate treatment. J. Hazard. Mater. 2020, 392, 122464. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gong, W.; Zheng, B.; Gao, L. Ni–Al bimetallic catalysts for preparation of multiwalled carbon nanotubes from polypropylene: Influence of the ratio of Ni/Al. Appl. Catal. B Environ. 2016, 181, 769–778. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Hu, Q.; Chen, Y.; Chen, H.; Williams, P.T. Carbon nanotubes from post-consumer waste plastics: Investigations into catalyst metal and support material characteristics. Appl. Catal. B Environ. 2021, 280, 119413. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P. Hydrogen production by steam gasification of polypropylene with various nickel catalysts. Appl. Catal. B Environ. 2009, 87, 152–161. [Google Scholar] [CrossRef]
- Zhao, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.J.; Ozcan, S. Plastic waste upcycling toward a circular economy. Chem. Eng. J. 2022, 428, 131928. [Google Scholar] [CrossRef]
- Munir, D.; Irfan, M.F.; Usman, M.R. Hydrocracking of virgin and waste plastics: A detailed review. Renew. Sustain. Energy Rev. 2018, 90, 490–515. [Google Scholar] [CrossRef]
- Kratish, Y.; Li, J.; Liu, S.; Gao, Y.; Marks, T.J. Polyethylene Terephthalate Deconstruction Catalyzed by a Carbon-Supported Single-Site Molybdenum-Dioxo Complex. Angew. Chem. Int. Ed. 2020, 59, 19857–19861. [Google Scholar] [CrossRef]
- Wu, P.; Lu, G.; Cai, C. Cobalt–molybdenum synergistic catalysis for the hydrogenolysis of terephthalate-based polyesters. Green Chem. 2021, 23, 8666–8672. [Google Scholar] [CrossRef]
- Weckhuysen, B.M. Creating value from plastic waste. Science 2020, 370, 400–401. [Google Scholar] [CrossRef]
- Petersen, H.A.; Myren, T.H.T.; O’Sullivan, S.J.; Luca, O.R. Electrochemical methods for materials recycling. Mater. Adv. 2021, 2, 1113–1138. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, D.; Wei, N. Enzyme discovery and engineering for sustainable plastic recycling. Trends Biotechnol. 2022, 40, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Blank, L.M.; Narancic, T.; Mampel, J.; Tiso, T.; O’Connor, K. Biotechnological upcycling of plastic waste and other non-conventional feedstocks in a circular economy. Curr. Opin. Biotechnol. 2020, 62, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Inderthal, H.; Tai, S.L.; Harrison, S.T. Non-Hydrolyzable Plastics—An Interdisciplinary Look at Plastic Bio-Oxidation. Trends Biotechnol. 2021, 39, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, J.; Khatri, M.; Arya, S.K. Recent insight into enzymatic degradation of plastics prevalent in the environment: A mini-review. Clean. Eng. Technol. 2021, 2, 100083. [Google Scholar] [CrossRef]
- Barth, M.; Oeser, T.; Wei, R.; Then, J.; Schmidt, J.; Zimmermann, W. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 2015, 93, 222–228. [Google Scholar] [CrossRef]
- Kim, H.T.; Kim, J.K.; Gil Cha, H.; Kang, M.J.; Lee, H.S.; Khang, T.U.; Yun, E.J.; Lee, D.-H.; Song, B.K.; Park, S.J.; et al. Biological Valorization of Poly(ethylene terephthalate) Monomers for Upcycling Waste PET. ACS Sustain. Chem. Eng. 2019, 7, 19396–19406. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Son, H.F.; Cho, I.J.; Joo, S.; Seo, H.; Sagong, H.-Y.; Choi, S.Y.; Lee, S.Y.; Kim, K.-J. Rational Protein Engineering of Thermo-Stable PETase from Ideonella sakaiensis for Highly Efficient PET Degradation. ACS Catal. 2019, 9, 3519–3526. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.C.; Wallace, S. Microbial synthesis of vanillin from waste poly(ethylene terephthalate). Green Chem. 2021, 23, 4665–4672. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Han, D.O.; Shim, K.I.; Kim, J.K.; Pelton, J.G.; Ryu, M.H.; Joo, J.C.; Han, J.W.; Kim, H.T.; Kim, K.H. One-Pot Chemo-bioprocess of PET Depolymerization and Recycling Enabled by a Biocompatible Catalyst, Betaine. ACS Catal. 2021, 11, 3996–4008. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, H.T.; Lee, M.-W.; Kim, K.-A.; Khang, T.U.; Song, H.M.; Park, S.J.; Joo, J.C.; Gil Cha, H. A chemo-microbial hybrid process for the production of 2-pyrone-4,6-dicarboxylic acid as a promising bioplastic monomer from PET waste. Green Chem. 2020, 22, 3461–3469. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Perrin, R.; Ullmann, C.; Persillon, C.; Phalip, V.; Avérous, L. Enzymatic recycling of thermoplastic polyurethanes: Synergistic effect of an esterase and an amidase and recovery of building blocks. Waste Manag. 2019, 85, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, S.T.; Nikodinovic-Runic, J.; Kaminsky, W.; Woods, T.; Babu, R.P.; Keely, C.M.; Blau, W.; O’Connor, K.E. Up-Cycling of PET (Polyethylene Terephthalate) to the Biodegradable Plastic PHA (Polyhydroxyalkanoate). Environ. Sci. Technol. 2008, 42, 7696–7701. [Google Scholar] [CrossRef]
- Tiso, T.; Narancic, T.; Wei, R.; Pollet, E.; Beagan, N.; Schröder, K.; Honak, A.; Jiang, M.; Kenny, S.T.; Wierckx, N.; et al. Towards bio-upcycling of polyethylene terephthalate. Metab. Eng. 2021, 66, 167–178. [Google Scholar] [CrossRef]
- Franden, M.A.; Jayakody, L.N.; Li, W.-J.; Wagner, N.J.; Cleveland, N.S.; Michener, W.E.; Hauer, B.; Blank, L.M.; Wierckx, N.; Klebensberger, J.; et al. Engineering Pseudomonas putida KT2440 for efficient ethylene glycol utilization. Metab. Eng. 2018, 48, 197–207. [Google Scholar] [CrossRef]
- Johnston, B.; Radecka, I.; Chiellini, E.; Barsi, D.; Ilieva, V.I.; Sikorska, W.; Musioł, M.; Zięba, M.; Chaber, P.; Marek, A.A.; et al. Mass Spectrometry Reveals Molecular Structure of Polyhydroxyalkanoates Attained by Bioconversion of Oxidized Polypropylene Waste Fragments. Polymers 2019, 11, 1580. [Google Scholar] [CrossRef]






| Composites | Processing Method | Obtained Properties | Applications | References |
|---|---|---|---|---|
| rPET/CuO NPs | Electrospinning and chemical precipitation techniques. | Photocatalytic activity efficiency for removing the methylene blue dye up to 99%. | Water treatment and filtration | [23] |
| rHDPE/CuO NPs | Melt mixing and compression molding. | Increased electron density, mass attenuation coefficient, and effective atomic number for γ-ray energies with point sources 356 keV from 133Ba, 662 keV from 137Cs, and 1332 keV from 60Co. | Radioactive source shielding | [24] |
| rLDPE/SiO2/TiO2 NPs | Melt extrusion, granulation, and compression molding. | Tensile strength of 8.4 MPa, and UV protection factor of 1500+. | UV shielding | [25] |
| rLDPE/PET/Al/ Graphite NPs | Shear milling, melt extrusion, and injection molding. | Thermal conductivity of 1.7 W/mK, and electrical conductivity of 10−10 S/cm. | Electronic packaging | [26] |
| rLDPE/PA/Al nanoflake | Powder mixing and compression molding. | Thermal conductivity in the range of 1.4–4.8 W/mK, and electrical conductivity of 10−13 S/cm. | Electronic packaging | [27] |
| rPS/SnO2 NPs | Thermally induced phase separation. | Photodegradation efficiency of rhodamine B dye under UV irradiation up to 98.2%. | Water treatment and filtration | [28] |
| rPS/TiO2 NPs/Al microparticles | Solution mixing and electrospinning. | Water contact angle of 157° (superhydrophobic), and daily water productivity of >1.35 L/m2. | Fog water-harvesting | [29] |
| Filament Material | Extrusion Conditions | 3D Printing Parameters | Mechanical Properties of Printed Structures | References |
|---|---|---|---|---|
| rPET/biochar composite | Single screw extrusion at 250 °C. | Bed temperature of 50 °C, nozzle temperature of 270 °C, layer height of 0.4 mm, print speed of 50 mm/s, nozzle diameter of 0.6 mm. | Tensile strengths in the range of 46–52 MPa, elastic modulus in the range of 0.7–0.9 GPa. | [37] |
| rPET/cellulose fiber composite | Twin screw extrusion with screw speed of 38–43 rpm, feed port at 200 °C, adjacent zone at 260 °C, main zones at 240 °C, die at 220 °C. | Nozzle temperature of 260 °C, print speed of 30 mm/s. | Impact resistance of 23.30 J/m, and impact strength of 2268 J/m2. | [38] |
| rPET/CCFs composite | Co-extrusion. | Bed temperature of 80 °C, nozzle temperature of 230 °C, layer height of 0.2 mm, print speed of 300 mm/s, nozzle diameter of 0.4 mm. | Tensile strength of 604.5 MPa, flexural strength of 318.6 MPa. | [39] |
| rHDPE/PP/PP-MAh blend | Single screw extrusion with screw speed of 20 rpm, feed port at 140 °C, adjacent zone at 150 °C, main zones at 160 °C, die at 155 °C. | Bed temperature of 105 °C, nozzle temperature of 215 °C. | Tensile yield stress of 4.78 MPa, strain of 38.1%. | [40] |
| rHDPE/CF composite | Twin screw extrusion with screw speed of 30 rpm, feed port at 180 °C, adjacent zone at 185 °C, main zones at 190 °C, die at 200 °C. | Bed temperature of 80 °C, nozzle temperature of 290 °C, nozzle diameter of 0.8 mm. | Tensile yield stress in the range of 18–21 MPa, tensile strengths in the range of 37–64 GPa. | [41] |
| rPP/cellulose composite | Twin screw extrusion with screw speed of 100 rpm, feed port at 140 °C, adjacent zone at 170 °C, main zones at 180 °C, die at 175 °C. | Bed temperature of 100 °C, nozzle temperature of 220 °C, layer height of 0.2 mm, print speed of 20–50 mm/s, nozzle diameter of 0.8 mm. | Tensile strengths in the range of 13–18 MPa, elastic modulus in the range of 1100–1500 MPa. | [42] |
| rPP/harakeke fibers, and rPP/hemp fibers composites | Twin screw extrusion with screw speed of 50 rpm, feed port at 150 °C, adjacent and main zones at 170 °C, die at 180 °C. | Nozzle temperature of 230 °C, print speed of 50 mm/min, nozzle diameter of 1.0 mm. | PP/harakeke fibers exhibited tensile strength and Young’s modulus in the range of 27–39 MPa and 1612–2767 MPa, respectively, whereas PP/hemp fibers were in the range of 28–38 MPa and 1683–2681 MPa. | [43] |
| rPP/CBS composite | Twin screw extrusion with screw speed in the range of 6–13 rpm, feed port at 175 °C, and the die at 190 °C. | Bed temperature of 90 °C, nozzle temperature of 250 °C, layer height of 0.25 mm, print speed of 60 mm/s, nozzle diameter of 0.8 mm. | Tensile strengths in the range of 8–15 MPa. | [44] |
| rPP/RH composite | Twin screw extrusion with screw speed of 9 rpm, feed port at 180 °C, adjacent zone at 185 °C, main zones at 190 °C, die at 195 °C. | Bed temperature of 80 °C, nozzle temperature of 240 °C, print speed of 60 mm/s, nozzle diameter of 0.8 mm. | Tensile strengths in the range of 5–14 MPa. | [45] |
| rPP/rPET, and rPP/rPS blend | Twin screw extrusion with screw speed of 25 rpm, feed port at 140 °C, adjacent zone at 170 °C, main zones at 240 °C, die at 245 °C. | Bed temperature of 100 °C, nozzle temperature of 260 °C, layer height of 0.2 mm, print speed of 20–50 mm/s, nozzle diameter of 0.5 mm. | PP/PET exhibited maximum tensile strength and elastic modulus of 24 MPa and 980 MPa, respectively, whereas PP/PS exhibited 23 MPa and 1459 MPa. | [46] |
| Plastic | Catalyst | Process | Products | Reference |
|---|---|---|---|---|
| HDPE | HZSM-5 zeolite | Pyrolysis | Ethylene | [52] |
| PE, PP, PS | Fe/Al2O3 | Pyrolysis | Amorphous carbon, carbon nanotubes, hydrogen | [53] |
| PP | Ni-Cu/La2O3 | Pyrolysis | Multiwalled carbon nanotubes, carbon nanofibers | [54] |
| LDPE | Fe-Mo/MgO | Pyrolysis | Carbon nanotubes, carbon nanofibers, graphene | [55] |
| PET, PE, PP | Ni/ZSM-5 | Gasification | Syngas | [56,57] |
| HDPE | Ni/CeO2–ZrO2 | Gasification | Hydrogen-rich syngas | [58] |
| HDPE | Ni-Fe/CNT-PC | Gasification | Hydrogen-rich syngas | [59] |
| PP | Ni/Al2O3 | Gasification | Hydrogen-rich syngas | [60] |
| LDPE | Pt/S-ZrO2 | Hydrogenolysis | Liquid fuels | [61] |
| LDPE | Pt/USY zeolite | Hydrogenolysis | Alkanes | [62] |
| PE | Pt/SrTiO3 | Hydrogenolysis | Lubricants | [63] |
| HDPE | Pt/SiO2/mSiO2 | Hydrogenolysis | Alkanes | [64] |
| LDPE | Pt/WZrO2 | Hydrogenolysis | Alkanes | [65] |
| LDPE | Pt/WO3/ZrO2/HY zeolite | Hydrogenolysis | Liquid fuels | [66] |
| PE, PP | Ru/C | Hydrogenolysis | Alkanes, liquid fuels, lubricants | [67,68,69] |
| PE, PP | Ru/CeO2 | Hydrogenolysis | Liquid fuels | [70] |
| PP | Ru/TiO2 | Hydrogenolysis | Lubricants | [71] |
| LDPE | Ru/WZrO2 | Hydrogenolysis | Alkanes | [72] |
| PET, PS | Ru/Nb2O5 | Hydrogenolysis | Arenes | [73] |
| LDPE, HDPE, PP | Ru/ZrO2 | Hydrogenolysis | Alkanes, liquid fuels | [74] |
| LDPE, PP, PS | Ru/FAU zeolite | Hydrogenolysis | Grid-compatible gas streams | [75] |
| PET | Co/TiO2 | Hydrogenolysis | Arenes | [76] |
| PET | CuNa/SiO2 | Hydrogenolysis | Alcohol, aromatics | [77] |
| PET | Pt/C, Ru-Cu/SiO2 | Tandem solvolysis–hydrogeneration | Cycloalkanes, aromatics | [78] |
| PE | Pt/γ-Al2O3 | Tandem hydrogenolysis–aromatization | Long-chain alkylaromatics | [79] |
| PP, PS | TiO2 | Photoreforming | Hydroxyl, carbonyl, and carbon-hydrogen groups | [80] |
| LDPE | ZnO2 | Photoreforming | Hydroperoxides, peroxides, carbonyl, and unsaturated groups | [81] |
| PET | CdS/CdOx | Photoreforming | Hydrogen | [82] |
| PET | CNx/Ni2P | Photoreforming | Hydrogen | [83] |
| PE | Pt/TiO2 | Tandem solvolysis–photoreforming | Alkene, alkane | [84] |
| PET | Pd/NF | Electroreforming | Hydrogen | [85] |
| PET | CoNi0.25P | Electroreforming | Hydrogen | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balu, R.; Dutta, N.K.; Roy Choudhury, N. Plastic Waste Upcycling: A Sustainable Solution for Waste Management, Product Development, and Circular Economy. Polymers 2022, 14, 4788. https://doi.org/10.3390/polym14224788
Balu R, Dutta NK, Roy Choudhury N. Plastic Waste Upcycling: A Sustainable Solution for Waste Management, Product Development, and Circular Economy. Polymers. 2022; 14(22):4788. https://doi.org/10.3390/polym14224788
Chicago/Turabian StyleBalu, Rajkamal, Naba Kumar Dutta, and Namita Roy Choudhury. 2022. "Plastic Waste Upcycling: A Sustainable Solution for Waste Management, Product Development, and Circular Economy" Polymers 14, no. 22: 4788. https://doi.org/10.3390/polym14224788