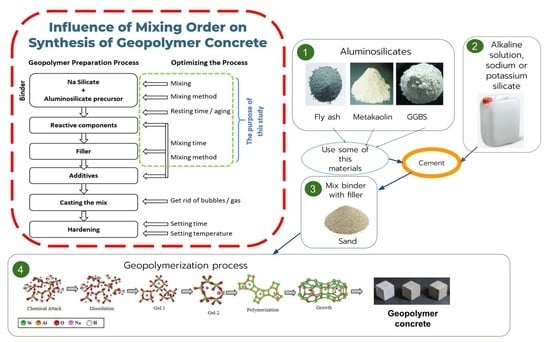
Influence of Mixing Order on the Synthesis of Geopolymer Concrete
Abstract
:1. Introduction
- Chemical composition;
- Structure;
- Morphology;
- Mechanical strength.
2. Materials and Methods
- Mix 1. FA was mixed with an alkaline solution for 10 min, and then followed by the addition of raw calcined kaolin clay (RCKC) that was mixed for 5 min, then GGBS was added, which had to be mixed for 3 min, and the last step was to add sand and mix for 3 min.
- Mix 2. All aluminosilicates (FA, RCKC, GGBS) were mixed with the alkaline activator in one step for 18 min and after that, the standard sand was introduced to the mixture which then was mixed for 3 min.
- Mix 3. All aluminosilicates (FA, RCKC, GGBS) and sand were mixed simultaneously with an alkaline activator for 21 min.
3. Results and Discussion
3.1. Compressive Strength
3.2. Microscopy
3.3. X-ray Diffraction Analysis
3.4. FTIR Spectroscopy
4. Conclusions
- (1)
- The mixing procedure of aluminosilicate precursors, which allows one ingredient to mix at a time, yields a higher degree of geopolymerization which results in a denser structure, and higher mechanical strength. The compressive strength of geopolymer concrete is increased by 31.7% and the flexural strength is increased by 20.3%. The compressive strength of geopolymers is inevitably correlated with the internal microstructure shown by SEM images, which in turn is formed by the polycondensation of multiple dissolution products of raw materials.
- (2)
- The simultaneous mixing of FA, RCKC, GGBS, and filler inhibits the reaction rate and reduces the average reactivity of the raw materials. The solid particles of kaolinite and FA that did not react during the dissolution of aluminosilicates are not completely connected with the matrix, and the residual pores and gaps in the structure around them can result in a decrease in mechanical performance. A more continuous and denser geopolymer gel phase is found in Mix 1, while Mix 2 and Mix 3 appear to have a more bulky, irregular structure with a larger distribution of pores and cracks.
- (3)
- The geopolymer mix design needs fully yield the potential of geopolymerization leading to the best performance and the highest mechanical strength. Therefore, the results of this study indicate that exploration of other mixing parameters such as mixing time, mixing speed (rotation per minute), and their influence on geopolymer performance are important for further research plans.
- (4)
- The separate mixing of aluminosilicates may not be practical in large-scale applications due to time-consuming factors, but it can be stated that the addition of aggregates after geopolymer paste preparation (Mix 2) provides superior mechanical strength and structure in the simultaneous mixing of geopolymer precursors and filler (Mix 3).
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vantyghem, G.; De Corte, W.; Shakour, E.; Amir, O. 3D printing of a post-tensioned concrete girder designed by topology optimization. Autom. Constr. 2020, 112, 103084. [Google Scholar] [CrossRef]
- Buswell, R.A.; Leal de Silva, W.R.; Jones, S.Z.; Dirrenberger, J. 3D printing using concrete extrusion: A roadmap for research. Cem. Concr. Res. 2018, 112, 37–49. [Google Scholar] [CrossRef]
- Khan, M.A. Mix suitable for concrete 3D printing: A review. Mater. Today Proc. 2020, 32, 831–837. [Google Scholar] [CrossRef]
- Kristombu Baduge, S.; Navaratnam, S.; Abu-Zidan, Y.; McCormack, T.; Nguyen, K.; Mendis, P.; Zhang, G.; Aye, L. Improving performance of additive manufactured (3D printed) concrete: A review on material mix design, processing, interlayer bonding, and reinforcing methods. Structures 2021, 29, 1597–1609. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Valente, M.; Sambucci, M.; Sibai, A. Geopolymers vs. Cement Matrix Materials: How Nanofiller Can Help a Sustainability Approach for Smart Construction Applications—A Review. Nanomaterials 2021, 11, 2007. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Saint-Quentin, France, 2008; Volume 171. [Google Scholar]
- Yang, K.-H.; Song, J.-K.; Song, K.-I. Assessment of CO2 reduction of alkali-activated concrete. J. Clean. Prod. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Sumajouw, M.D.J.; Rangan, B.V. Low-Calcium Fly Ash-Based Geopolymer Concrete: Reinforced Beams and Columns; Curtin University of Technology: Perth, Australia, 2006. [Google Scholar]
- Kong, D.L.Y.; Sanjayan, J.G. Damage behavior of geopolymer composites exposed to elevated temperatures. Cem. Concr. Compos. 2008, 30, 986–991. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening evolution of geopolymers from setting to equilibrium: A review. Cem. Concr. Compos. 2020, 114, 103729. [Google Scholar] [CrossRef]
- Egov.kz. Public Services and Online Information. Available online: https://egov.kz/cms/en/articles/ecology/waste_reduction_recycling_and_reuse (accessed on 26 September 2022).
- PrimeMinister.kz. Production Volume for Building Materials Increased 3 Times. Prime Minister Press Service of the Republic of Kazakhstan. 2020. Available online: https://primeminister.kz/ru/news/v-kazahstane-obemproizvodstva-stroitelnyh-materialov-uvelichilsya-v-33-raza-miir-rk-2161319 (accessed on 1 August 2022).
- Zhou, Y.; Weng, Y.; Li, L.; Hu, B.; Huang, X.; Zhu, Z. Recycled GFRP Aggregate Concrete Considering Aggregate Grading: Compressive Behavior and Stress-Strain Modeling. Polymers 2022, 14, 581. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Hu, Z.; Zhou, Y.; Wu, Y.; Zhou, X.; Hu, B.; Guo, M. Improving recycled aggregate concrete by compression casting and nano-silica. Nanotechnol. Rev. 2022, 11, 1273–1290. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, H.; Hu, Z.; Qiu, Y.; Guo, M.; Huang, X.; Hu, B. Ductile, durable, and reliable alternative to FRP bars for reinforcing seawater sea-sand recycled concrete beams: Steel/FRP composite bars. Constr. Build. Mater. 2021, 269, 121264. [Google Scholar] [CrossRef]
- KazTAG. Prices for Construction Materials Increased by 25% in Kazakhstan. 2021. Available online: https://kaztag.kz/en/news/prices-for-construction-materials-increased-by-25-in-kazakhstan (accessed on 16 September 2021).
- Nguyen, K.T.; Nguyen, Q.D.; Le, T.A.; Shin, J.; Lee, K. Analyzing the compressive strength of green fly ash based geopolymer concrete using experiment and machine learning approaches. Constr. Build. Mater. 2020, 247, 118581. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mostafa, R.R.; Mohammed, A.; Sihag, P.; Qadir, A. Support vector regression (SVR) and grey wolf optimization (GWO) to predict the compressive strength of GGBFS-based geopolymer concrete. Neural Comput. Appl. 2022, 17, 1–18. [Google Scholar] [CrossRef]
- Raza, M.H.; Zhong, R.Y. A sustainable roadmap for additive manufacturing using geopolymers in construction industry. Resour. Conserv. Recycl. 2022, 186, 106592. [Google Scholar] [CrossRef]
- Imtiaz, L.; Rehman, S.K.U.; Ali Memon, S.; Khizar Khan, M.; Faisal Javed, M. A Review of Recent Developments and Advances in Eco-Friendly Geopolymer Concrete. Appl. Sci. 2020, 10, 7838. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Mahmood, A.H.; Foster, S.J.; Castel, A. Effects of mixing duration on engineering properties of geopolymer concrete. Constr. Build. Mater. 2021, 303, 124449. [Google Scholar] [CrossRef]
- Hardjito, D.; Sumajouw, D.M.J. Introducing fly ash-based geopolymer concrete: Manufacture and engineering properties. In Proceedings of the 30th Conference on Our World in Concrete & Structures, Singapore, 23–24 August 2005. [Google Scholar]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S.J. Effect of Alumina Release Rate on the Mechanism of Geopolymer Gel Formation. Chem. Mater. 2010, 22, 5199–5208. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.M.; Palomo, A.; Lopez-Hombrados, C. Engineering Properties of Alkali-Activated Fly Ash Concrete. ACI Mater. J. 2006, 103, 106. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Chithiraputhiran, S.; Neithalath, N. Isothermal reaction kinetics and temperature dependence of alkali activation of slag, fly ash and their blends. Constr. Build. Mater. 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Structural reorganisation of class F fly ash in alkaline silicate solutions. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 49–66. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Lukey, G.C.; Van Deventer, J.S.J. The stabilisation of mine tailings by reactive geopolymerisation. Publ. Australas. Inst. Min. Metall. 2000, 5, 363–371. [Google Scholar]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Schwartzman, A. The potential use of geopolymeric materials to immobilise toxic metals: Part II. Material and leaching characteristics. Miner. Eng. 1999, 12, 75–91. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; van Deventer, J.S.J.; Lukey, G.C. Effect of Alkali Metals on the Preferential Geopolymerization of Stilbite/Kaolinite Mixtures. Ind. Eng. Chem. Res. 2001, 40, 3749–3756. [Google Scholar] [CrossRef]
- ASTM C618-19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- Yip, C.K.; Lukey, G.C.; van Deventer Dean, J.S.J. Effect of Blast Furnace Slag Addition on Microstructure and Properties of Metakaolinite Geopolymeric Materials. In Advances in Ceramic Matrix Composites IX; The American Ceramic Society: Columbus, OH, USA, 2006; pp. 187–209. [Google Scholar]
- ASTM C39/C39M-16; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- ASTM C293/C293M-16; Standard Test Method for Flexural Strength of Concrete (Using Simple Beam With Center-Point Loading). ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- Yuan, L.; Ma, Y.; Zhang, J.; Men, J.; Sun, T.; Zhao, H.; Wu, H.; Wang, H.; Dai, S. Orthogonal analysis and mechanism of compressive strength and microstructure of the metakaolin-fly ash geopolymer. Case Stud. Constr. Mater. 2022, 17, e01154. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Nenadović, S.; Gulicovski, J.; Mirković, M.; Kljajević, L.; Bošković, I.; Vukčević, M.; Nenadović, M. Structural, Mechanical and Chemical Properties of Low Content Carbon Geopolymer. Sustainability 2022, 14, 4885. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Do Geopolymers Actually Contain Nanocrystalline Zeolites? A Reexamination of Existing Results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Sha, D.; Pan, B.; Sun, Y. A novel raw material for geopolymers: Coal-based synthetic natural gas slag. J. Clean. Prod. 2020, 262, 121238. [Google Scholar] [CrossRef]
- Sha, D.; Pan, B.; Sun, Y. Investigation on mechanical properties and microstructure of coal-based synthetic natural gas slag (CSNGS) geopolymer. Constr. Build. Mater. 2020, 259, 119793. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Li, J.; Tao, Y.; Zhuang, E.; Cui, X.; Yu, K.; Yu, B.; Boluk, Y.; Bindiganavile, V.; Chen, Z.; Yi, C. Optimal amorphous oxide ratios and multifactor models for binary geopolymers from metakaolin blended with substantial sugarcane bagasse ash. J. Clean. Prod. 2022, 377, 134215. [Google Scholar] [CrossRef]
- Silva, A.M.B.; Queiroz, C.M.; Agathopoulos, S.; Correia, R.N.; Fernandes, M.H.V.; Oliveira, J.M. Structure of SiO2–MgO–Na2O glasses by FTIR, Raman and 29Si MAS NMR. J. Mol. Struct. 2011, 986, 16–21. [Google Scholar] [CrossRef]











| Chemical Composition (wt. %) | FA | GGBS | RCKC |
|---|---|---|---|
| SiO2 | 65.605 | 31.0106 | 63.0988 |
| TiO2 | 1.2755 | 0.6936 | 0.5504 |
| Al2O3 | 25.0715 | 8.9084 | 28.9502 |
| MgO | 0.5323 | 10.7629 | 0.2343 |
| CaO | 2.5266 | 26.6471 | 1.9797 |
| Na2O | 0.6127 | 0.6764 | 0.7943 |
| K2O | 0.485 | 0.8022 | 0.9348 |
| P2O5 | 0.2941 | 0.1057 | 0.1057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhametkaliyev, T.; Ali, M.H.; Kutugin, V.; Savinova, O.; Vereschagin, V. Influence of Mixing Order on the Synthesis of Geopolymer Concrete. Polymers 2022, 14, 4777. https://doi.org/10.3390/polym14214777
Mukhametkaliyev T, Ali MH, Kutugin V, Savinova O, Vereschagin V. Influence of Mixing Order on the Synthesis of Geopolymer Concrete. Polymers. 2022; 14(21):4777. https://doi.org/10.3390/polym14214777
Chicago/Turabian StyleMukhametkaliyev, Timur, Md. Hazrat Ali, Viktor Kutugin, Olesya Savinova, and Vladimir Vereschagin. 2022. "Influence of Mixing Order on the Synthesis of Geopolymer Concrete" Polymers 14, no. 21: 4777. https://doi.org/10.3390/polym14214777