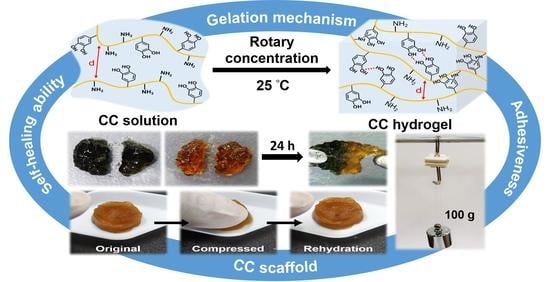
Gelation and the Self-Healing Behavior of the Chitosan–Catechol Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Chitosan–Catechol (CC)
2.3. Preparation of CC Hydrogels
2.4. Rheological Properties of CC Hydrogels
2.5. Coherent SAXS and In Situ SAXS
2.6. Macroscopic Self-Healing Property and Injectability of the CC Hydrogel
2.7. Osmotic-Responsive Properties of the CC Hydrogel
2.8. Adhesive Properties of CC Hydrogels
2.9. Preparation and Characterization of CC Scaffolds
2.10. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of CC
3.2. Characterization of CC Hydrogels
3.3. Strain-Dependent Performance of the CC Hydrogel
3.4. Injectability and Self-Healing Properties of the CC Hydrogel
3.5. Osmotic-Responsive Behavior and Adhesive Properties of the Hydrogels
3.6. Preparation and Characterization of the CC Scaffolds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, P. Antimicrobial chitosan conjugates: Current synthetic strategies and potential applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorganic Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Hoang, D.Q.; Nguyen, N.D.; Nguyen, Q.H.; Nguyen, D.H. Preparation, characterization, and antioxidant activity of water-soluble oligochitosan. Green Process. Synth. 2017, 6, 461–468. [Google Scholar] [CrossRef]
- Knight, D.K.; Shapka, S.N.; Amsden, B.G. Structure, depolymerization, and cytocompatibility evaluation of glycol chitosan. J. Biomed. Mater. Res. A. 2007, 83, 787–798. [Google Scholar] [CrossRef]
- Sashiwa, H.; Kawasaki, N.; Nakayama, A.; Muraki, E.; Yamamoto, N.; Aiba, S.-i. Chemical modification of chitosan. 14: Synthesis of water-soluble chitosan derivatives by simple acetylation. Biomacromolecules 2002, 3, 1126–1128. [Google Scholar] [CrossRef]
- Chen, X.-G.; Park, H.-J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, J.H.; Lee, D.Y.; Lee, H. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. 2013, 1, 783–790. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Annabi, N.; Rana, D.; Sani, E.S.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef]
- Pellá, M.C.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.A.; Taylor, K.E. Chitosan gels, 3. The formation of gels by reaction of chitosan with glutaraldehyde. Die Makromol. Chem. Macromol. Chem. Phys. 1989, 190, 951–960. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.-H.; Chuang, W.-T.; Dai, N.-T.; Hsu, S.-h. Biomimetic strain-stiffening in chitosan self-healing hydrogels. ACS Appl. Mater. Interfaces 2022, 14, 16032–16046. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, J.; Huh, K.M.; Lee, S.H.; Lee, H. Toxicity-Attenuated Glycol Chitosan Adhesive Inspired by Mussel Adhesion Mechanisms. Adv. Healthc. Mater. 2019, 8, 1900275. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Gebbie, M.A.; Wei, W.; Schrader, A.M.; Cristiani, T.R.; Dobbs, H.A.; Idso, M.; Chmelka, B.F.; Waite, J.H.; Israelachvili, J.N. Tuning underwater adhesion with cation–π interactions. Nat. Chem. 2017, 9, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The chemistry behind catechol-based adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.K.; Zhao, B.; Pinnaratip, R. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef]
- Xu, L.Q.; Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Dopamine-induced reduction and functionalization of graphene oxide nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Wang, Y.; Long, Y.; Gao, H.; Zhang, X.; Zhao, N.; Cai, Y.; Xu, J. Mussel-inspired chemistry for robust and surface-modifiable multilayer films. Langmuir 2011, 27, 13684–13691. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, K.D.; Pyo, K.B.; Park, S.Y.; Lee, H. Catechol-grafted poly (ethylene glycol) for PEGylation on versatile substrates. Langmuir 2010, 26, 3790–3793. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yang, K.; Kang, B.; Lee, C.; Song, I.T.; Byun, E.; Park, K.I.; Cho, S.W.; Lee, H. Hyaluronic acid catechol: A biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.-I.; Lee, H.; Cho, S.-W. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Srivastava, A. Robust, self-healing hydrogels synthesised from catechol rich polymers. J. Mater. Chem. B 2015, 3, 899–910. [Google Scholar] [CrossRef]
- Lin, S.-H.; Papadakis, C.M.; Kang, J.-J.; Lin, J.-M.; Hsu, S.-h. Injectable phenolic-chitosan self-healing hydrogel with hierarchical micelle architectures and fast adhesiveness. Chem. Mater. 2021, 33, 3945–3958. [Google Scholar] [CrossRef]
- Serizawa, T.; Wakita, K.; Akashi, M. Rapid deswelling of porous poly (N-isopropylacrylamide) hydrogels prepared by incorporation of silica particles. Macromolecules 2002, 35, 10–12. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; Fan, B.; Xu, Y.; Liang, B. A novel sodium carboxymethylcellulose/poly (N-isopropylacrylamide)/Clay semi-IPN nanocomposite hydrogel with improved response rate and mechanical properties. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1546–1555. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Scher, J.; Muniglia, L. Enzymatic synthesis of chitosan derivatives and their potential applications. J. Mol. Catal. B Enzym. 2015, 112, 25–39. [Google Scholar] [CrossRef]
- Nishimura, S.; Kohgo, O.; Kurita, K.; Kuzuhara, H. Chemospecific manipulations of a rigid polysaccharide: Syntheses of novel chitosan derivatives with excellent solubility in common organic solvents by regioselective chemical modifications. Macromolecules 1991, 24, 4745–4748. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, C.-W.; Chang, S.-W.; Hsu, S.-h. An injectable, self-healing phenol-functionalized chitosan hydrogel with fast gelling property and visible light-crosslinking capability for 3D printing. Acta Biomater. 2021, 122, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Ryu, J.H.; Lee, D.; Lee, H. Freeze–Thawing-Induced Macroporous Catechol Hydrogels with Shape Recovery and Sponge-like Properties. ACS Biomater. Sci. Eng. 2021, 7, 4318–4329. [Google Scholar] [CrossRef]
- Waite, J.H. The phylogeny and chemical diversity of quinone-tanned glues and varnishes. Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 97, 19–29. [Google Scholar] [CrossRef]
- Yang, J.; Saggiomo, V.; Velders, A.H.; Cohen Stuart, M.A.; Kamperman, M. Reaction pathways in catechol/primary amine mixtures: A window on crosslinking chemistry. PLoS ONE 2016, 11, e0166490. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.C.; Dougherty, D.A. The cation−π interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chuang, W.-T.; Hsu, S.-h. Gelation mechanism and structural dynamics of chitosan self-healing hydrogels by in situ SAXS and coherent X-ray scattering. ACS Macro Lett. 2019, 8, 1449–1455. [Google Scholar] [CrossRef]
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 394, 124888. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D. A universal soaking strategy to convert composite hydrogels into extremely tough and rapidly recoverable double-network hydrogels. Adv. Mater. 2016, 28, 7178–7184. [Google Scholar] [CrossRef]
- Fang, W.; Yang, L.; Hong, L.; Hu, Q. A chitosan hydrogel sealant with self-contractile characteristic: From rapid and long-term hemorrhage control to wound closure and repair. Carbohydr. Polym. 2021, 271, 118428. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yoo, H.Y.; Huang, J.; Lee, Y.; Park, S.; Park, Y.; Jin, S.; Jung, Y.M.; Zeng, H.; Hwang, D.S. Salt triggers the simple coacervation of an underwater adhesive when cations meet aromatic π electrons in seawater. ACS Nano 2017, 11, 6764–6772. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Park, S.-G.; Oh, B.-C.; Kim, K.; Jo, S.; Lee, M.S.; Oh, S.S.; Hong, S.-H.; Shin, E.-C.; Kim, K.-S. Complete prevention of blood loss with self-sealing haemostatic needles. Nat. Mater. 2017, 16, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling behaviors of pH-and salt-responsive cellulose-based hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. Rsc Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Ayyadurai, N.; Yun, H.; Choi, Y.S.; Hwang, B.H.; Huang, J.; Lu, Q.; Zeng, H.; Cha, H.J. In Vivo residue-specific dopa-incorporated engineered mussel bioglue with enhanced adhesion and water resistance. Angew. Chem. 2014, 126, 13578–13582. [Google Scholar] [CrossRef]
- Choi, Y.C.; Choi, J.S.; Jung, Y.J.; Cho, Y.W. Human gelatin tissue-adhesive hydrogels prepared by enzyme-mediated biosynthesis of DOPA and Fe3+ ion crosslinking. J. Mater. Chem. B 2014, 2, 201–209. [Google Scholar] [CrossRef]
- Kim, S.; Faghihnejad, A.; Lee, Y.; Jho, Y.; Zeng, H.; Hwang, D.S. Cation–π interaction in DOPA-deficient mussel adhesive protein mfp-1. J. Mater. Chem. B 2015, 3, 738–743. [Google Scholar] [CrossRef]









| Hydrogels | Solid Contents (wt%) | Storage Modulus (G′, Pa) |
|---|---|---|
| CC0.2 | 0.2 | 0.3 |
| CC0.5 | 0.5 | 0.4 |
| CC1.0 | 1.0 | 20.6 |
| CC1.5 | 1.5 | 45.7 |
| CC2.0 | 2.0 | 52.8 |
| Scaffolds | Lyophilized Hydrogels | Porosity (%) | Swelling Ratio (%) | Compressive Modulus (kPa) |
|---|---|---|---|---|
| CCs1 | CC1.5 | 96.6 ± 1.3 | 6646 ± 127 | 7.13 ± 0.07 |
| CCs2 | CC2.0 | 98.4 ± 0.9 | 9656 ± 93 | 4.19 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.-T.; Cheng, Q.-P.; Xu, J.; Lin, S.-H.; Lin, J.-M.; Hsu, S.-h. Gelation and the Self-Healing Behavior of the Chitosan–Catechol Hydrogel. Polymers 2022, 14, 4614. https://doi.org/10.3390/polym14214614
Lan Y-T, Cheng Q-P, Xu J, Lin S-H, Lin J-M, Hsu S-h. Gelation and the Self-Healing Behavior of the Chitosan–Catechol Hydrogel. Polymers. 2022; 14(21):4614. https://doi.org/10.3390/polym14214614
Chicago/Turabian StyleLan, Yu-Ting, Qian-Pu Cheng, Junpeng Xu, Shih-Ho Lin, Jhih-Min Lin, and Shan-hui Hsu. 2022. "Gelation and the Self-Healing Behavior of the Chitosan–Catechol Hydrogel" Polymers 14, no. 21: 4614. https://doi.org/10.3390/polym14214614