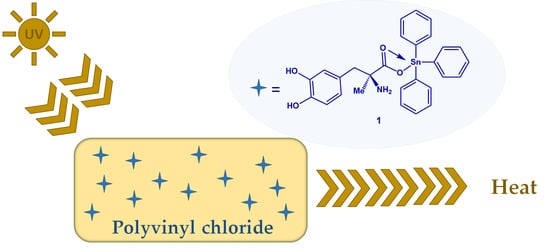
Synthesis of Methyldopa–Tin Complexes and Their Applicability as Photostabilizers for the Protection of Polyvinyl Chloride against Photolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Samples Preparation
2.2. Methods
2.3. Synthesis of 1 and 2
2.4. Synthesis of 3–5
2.5. Preparation of PVC Films
2.6. Assessment of IR Spectra
2.7. Assessment of Weight Loss
2.8. Assessment of Average Molecular Weight (MV)
3. Results and Discussion
3.1. Synthesis of Complexes 1–5
3.2. The EDX Spectroscopy of PVC Films
3.3. IR Spectral Study of PVC Films
3.4. Weight Loss Investigation of PVC Films
3.5. Viscosity Average Molecular Weight (MV) Study of PVC Films
3.6. Surface Assessment of the Irradiated PVC Films
3.7. Suggested Mechanisms for Photostabilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yu, X.; Cheng, Z. Research on the application of synthetic polymer materials in contemporary public art. Polymers 2022, 14, 1208. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Lovell, P.A. Introduction to Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 76. [Google Scholar] [CrossRef]
- Neuba, L.D.M.; Junio, R.F.P.; Ribeiro, M.P.; Souza, A.T.; Lima, E.D.S.; Filho, F.D.C.G.; Figueiredo, A.B.-H.D.S.; Braga, F.D.O.; De Azevedo, A.R.G.; Monteiro, S.N. Promising mechanical, thermal, and ballistic properties of novel epoxy composites reinforced with Cyperus malaccensis sedge fiber. Polymers 2020, 12, 1776. [Google Scholar] [CrossRef] [PubMed]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Al-Khazrajy, O.S.A.; Abdallh, M.; Alanazi, S.A. Modifications of polymers through the addition of ultraviolet absorbers to reduce the aging effect of accelerated and natural irradiation. Polymers 2022, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, A.A.; Noor, N.H.M.; Serrà, A.; Mohamad Ibrahim, M.N. Advances and challenges in developing efficient graphene oxide-based ZnO photocatalysts for dye photo-oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [Green Version]
- Lieberzeit, P.; Bekchanov, D.; Mukhamediev, M. Polyvinyl chloride modifications, properties, and applications: Review. Polym. Adv. Technol. 2022, 33, 1809–1820. [Google Scholar] [CrossRef]
- Keane, M.A. Catalytic conversion of waste plastics: Focus on waste PVC. J. Chem. Technol. Biotechnol. 2007, 82, 787–795. [Google Scholar] [CrossRef]
- Ma, Y.-F.; Liao, S.-L.; Li, Q.-G.; Guan, Q.; Jia, P.-Y.; Zhou, Y.-H. Physical and chemical modifications of poly(vinyl chloride) materials to prevent plasticizer migration—Still on the run. React. Funct. Polym. 2019, 147, 104458. [Google Scholar] [CrossRef]
- Starnes, W.H., Jr. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.B.; Kumosa, M. Particle removal mechanisms in synergistic aging of polymers and glass reinforced polymer composites under combined UV and water. Compos. Sci. Technol. 2017, 153, 273–281. [Google Scholar] [CrossRef]
- Lewandowski, K.; Skórczewska, K. A Brief review of poly(Vinyl chloride) (PVC) recycling. Polymers 2022, 14, 3035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wen, S.; Feng, Q.; Wang, H. Enhanced sulfidization of azurite surfaces by ammonium phosphate and its effect on flotation. Int. J. Miner. Metall. Mater. 2022, 29, 1150–1160. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Yang, B.; Feng, Q.; Liu, D. Enhanced sulfidization flotation mechanism of smithsonite in the synergistic activation system of copper–ammonium species. Miner. Eng. 2022, 187, 107796. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV degradation model for polymers and polymer matrix composites. Polym. Degrad. Stab. 2018, 154, 203–210. [Google Scholar] [CrossRef]
- Liu, J.; Lv, Y.; Luo, Z.; Wang, H.; Wei, Z. Molecular chain model construction, thermo-stability, and thermo-oxidative degradation mechanism of poly(vinyl chloride). RSC Adv. 2016, 6, 31898–31905. [Google Scholar] [CrossRef]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar] [CrossRef]
- Zheng, X.-G.; Tang, L.-H.; Zhang, N.; Gao, Q.-H.; Zhang, C.-F.; Zhu, Z.-B. Dehydrochlorination of PVC materials at high temperature. Energy Fuels 2003, 17, 896–900. [Google Scholar] [CrossRef]
- Valko, L.; Klein, E.; Kovařıík, P.; Bleha, T.; Šimon, P. Kinetic study of thermal dehydrochlorination of poly(vinyl chloride) in the presence of oxygen: III. Statistical thermodynamic interpretation of the oxygen catalytic activity. Eur. Polym. J. 2001, 37, 1123–1132. [Google Scholar] [CrossRef]
- Gao, A.X.; Bolt, J.D.; Feng, A.A. Role of titanium dioxide pigments in outdoor weathering of rigid PVC. Plast. Rubber Compos. 2008, 37, 397–402. [Google Scholar] [CrossRef]
- Chai, R.D.; Zhang, J. Synergistic effect of hindered amine light stabilizers/ultraviolet absorbers on the polyvinyl chloride/powder nitrile rubber blends during photodegradation. Polym. Eng. Sci. 2013, 53, 1760–1769. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Braun, D. Recycling of PVC. Prog. Polym. Sci. 2002, 27, 2171–2195. [Google Scholar] [CrossRef]
- Porta, M.; Zumeta, E. Implementing the Stockholm treaty on persistent organic pollutants. Occup. Environ. Med. 2002, 59, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, R.F. Mixed metal vinyl stabilizer synergism. II: Reactions with zinc replacing cadmium. J. Vinyl Addit. Technol. 1990, 12, 142–145. [Google Scholar] [CrossRef]
- Li, D.; Xie, L.; Fu, M.; Zhang, J.; Indrawirawan, S.; Zhang, Y.; Tang, S. Synergistic effects of lanthanum-pentaerythritol alkoxide with zinc stearates and with beta-diketone on the thermal stability of poly(vinyl chloride). Polym. Degrad. Stab. 2015, 114, 52–59. [Google Scholar] [CrossRef]
- Fu, M.; Li, D.; Liu, H.; Ai, H.; Zhang, Y.; Zhang, L. Synergistic effects of zinc-mannitol alkoxide with calcium/zinc stearates and with β-diketone on thermal stability of rigid poly(vinyl chloride). J. Polym. Res. 2016, 23, 13. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Alotaibi, M.H.; Star, H.A.; Ahmed, A.A. Influence of polyphosphates on the physicochemical properties of poly(vinyl chloride) after irradiation with ultraviolet light. Polymers 2020, 12, 193. [Google Scholar] [CrossRef]
- Schiller, M. PVC Additives: Performance, Chemistry, Developments, and Sustainability; Carl Hanser Verlag: Munich, Germany, 2015. [Google Scholar]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide particles on the surface morphology and the mechanical properties of PVC composites during QUV accelerated weathering. Polym. Compos. 2016, 37, 3391–3397. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohammed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide on chemical and molecular changes in PVC sidings during QUV accelerated weathering. Polym. Degrad. Stab. 2014, 104, 33–39. [Google Scholar] [CrossRef]
- Annuar, S.N.S.; Kamaludin, N.F.; Awang, N.; Chan, K.M. Cellular basis of organotin(IV) derivatives as anticancer metallodrugs: A review. Front. Chem. 2021, 9, 657599. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, J.O.; Onwudiwe, D.C. Organotin(IV) dithiocarbamate complexes: Chemistry and biological activity. Molecules 2018, 23, 2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Farina, Y.; Mun, L.K.; Rajab, N.F.; Awang, N. Syntheses, characterization, X-ray diffraction studies and in vitro antitumor activities of diorganotin(IV) derivatives of bis(p-substituted-N-methylbenzylaminedithiocarbamates). Polyhedron 2015, 85, 754–760. [Google Scholar] [CrossRef]
- Niu, L.; Li, Y.; Li, Q. Medicinal properties of organotin compounds and their limitations caused by toxicity. Inorg. Chim. Acta 2014, 423, 2–13. [Google Scholar] [CrossRef]
- Pellerito, C.; Nagy, L.; Pellerito, L.; Szorcsik, A. Biological activity studies on organotin (IV)n+ complexes and parent compounds. J. Organomet. Chem. 2006, 691, 1733–1747. [Google Scholar] [CrossRef]
- Davies, A.G. Organotin Chemistry, 2nd ed.; Wiley-VCH: Weinheim, Germany; John Wiley: Chichester, UK, 2004. [Google Scholar]
- Mah, G.T.; Tejani, A.M.; Musini, V.M. Methyldopa for primary hypertension. Cochrane Database Syst Rev. 2009, 2009, CD003893. [Google Scholar] [CrossRef]
- Gardette, J.L.; Gaumet, S.; Lemaire, J. Photooxidation of poly(vinyl chloride). 1. A reexamination of the mechanism. Macromolecules 1989, 22, 2576–2581. [Google Scholar] [CrossRef]
- Chaochanchaikul, K.; Rosarpitak, V.; Sombatsompop, N. Photodegradation profiles of PVC compound and wood/PVC composites under UV weathering. Express Polym. Lett. 2013, 7, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Pi, H.; Xiong, Y.; Guo, S. The kinetic studies of elimination of HCl during thermal decomposition of PVC in the presence of transition metal oxides. Polym. Plast. Technol. Eng. 2005, 44, 275–288. [Google Scholar] [CrossRef]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Pospíšil, J.; Nešpurek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Pepperl, G. Molecular weight distribution of commercial PVC. J. Vinyl Addit. Technol. 2000, 6, 88–92. [Google Scholar] [CrossRef]
- Alcock, N.W.; Culver, J.; Roe, S.M. Secondary bonding. Part 15. Influence of lone pairs on coordination: Comparison of diphenyl-tin (IV) and –tellurium (IV) carboxylates and dithiocarbamates. J. Chem. Soc. Dalton Trans. 1992, 9, 1477–1484. [Google Scholar] [CrossRef]
- Barroso-Flores, J.; Cea-Olivares, R.; Toscano, R.A.; Cogordan, J.A. Synthesis of the anisobidentate compound bis(2-amino-cyclopent-1-ene-carbodithioate)diethyltin (IV). Experimental and theoretical study. J. Organomet. Chem. 2004, 689, 2096–2102. [Google Scholar] [CrossRef]
- Dragnevski, K.I.; Donald, A.M.; Clarke, S.M.; Maltby, A. Novel applications of ESEM and EDX for the study of molecularly thin amide monolayers on polymer films. Colloid. Surf. A 2009, 337, 47–51. [Google Scholar] [CrossRef]
- Abd Mutalib, M.; Rahman, M.A.; Othman, M.H.D.; Ismail, A.F.; Jaafar, J. Scanning electron microscopy (SEM) and energy-dispersive X-Ray (EDX) spectroscopy. In Membrane Characterization; Hilal, N., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Oxford, UK, 2017; Volume Chapter 9, pp. 161–179. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci. Total Environ. 2017, 603–604, 616–626. [Google Scholar] [CrossRef]
- Karayıldırım, T.; Yanık, J.; Yüksel, M.; Saglam, M.; Haussmann, M. Degradation of PVC containing mixtures in the presence of HCl fixators. J. Polym. Environ. 2005, 13, 365–379. [Google Scholar] [CrossRef]
- Nief, O.A. Photostabilization of polyvinyl chloride by some new thiadiazole derivatives. Eur. J. Chem. 2015, 6, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Jafari, A.J.; Donaldson, J.D. Determination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper(II) oxide and copper(II) chloride. J. Chem. 2009, 6, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Skillicorn, D.E.; Perkins, G.G.A.; Slark, A.; Dawkins, J.V. Molecular weight and solution viscosity characterization of PVC. J. Vinyl Addit. Technol. 1993, 15, 105–108. [Google Scholar] [CrossRef]
- See, C.H.; O’Haver, J. Atomic force microscopy characterization of ultrathin polystyrene films formed by admicellar polymerization on silica disks. J. Appl. Polym. Sci. 2003, 89, 36–46. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Ahmadi, M.; Mirmohseni, A.; Taseidifar, M.; Naebe, M. The effects of UV light on the chemical and mechanical properties of a transparent epoxy-diamine system in the presence of an organic UV absorber. Materials 2017, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, N.; Andreasson, E.; Kao-Walter, S. SEM observations of a metal foil laminated with a polymer film. Procedia Mater. Sci. 2014, 3, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Kayyarapu, B.; Kumar, M.Y.; Mohommad, H.B.; Neeruganti, G.O.; Chekuri, R. Structural, thermal and optical properties of pure and Mn2+ doped poly(vinyl chloride) films. Mater. Res. 2016, 19, 1167–1175. [Google Scholar] [CrossRef] [Green Version]
- Hadi, A.G.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Jawad, K.; Alotaibi, M.H.; Hashim, H. Long-term effect of ultraviolet irradiation on poly(vinyl chloride) films containing naproxen diorganotin(IV) complexes. Molecules 2019, 24, 2396. [Google Scholar] [CrossRef] [Green Version]
- Mousa, O.G.; El-Hiti, G.A.; Baashen, M.A.; Bufaroosha, M.; Ahmed, A.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Synthesis of carvedilol-organotin complexes and their effects on reducing photodegradation of poly(vinyl chloride). Polymers 2021, 13, 500. [Google Scholar] [CrossRef]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; El-Hiti, G.A.; Yousif, E.; Ahmed, A.A.; Ahmed, D.S.; Alotaibi, M.H. Protection of poly(vinyl chloride) films against photodegradation using various valsartan tin complexes. Polymers 2020, 12, 969. [Google Scholar] [CrossRef] [Green Version]
- Hadi, A.G.; Jawad, K.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Photostabilization of poly(vinyl chloride) by organotin(IV) compounds against photodegradation. Molecules 2019, 24, 3557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaseen, A.A.; Yousif, E.; Al-Tikrity, E.T.B.; El-Hiti, G.A.; Kariuki, B.M.; Ahmed, D.S.; Bufaroosha, M. FTIR, weight, and surface morphology of poly(vinyl chloride) doped with tin complexes containing aromatic and heterocyclic moieties. Polymers 2021, 13, 3264. [Google Scholar] [CrossRef] [PubMed]
- Fadhil, M.; Yousif, E.; Ahmed, D.S.; Mohammed, A.; Hashim, H.; Ahmed, A.; Kariuki, B.M.; El-Hiti, G.A. Synthesis of new norfloxacin–tin complexes to mitigate the effect of ultraviolet-visible irradiation in polyvinyl chloride films. Polymers 2022, 14, 2812. [Google Scholar] [CrossRef] [PubMed]
- Fadhil, M.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Synthesis and application of levofloxacin–tin complexes as new photostabilizers for polyvinyl chloride. Polymers 2022, 14, 3720. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin(IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef] [Green Version]
- Ghani, H.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Tin complexes of 4-(benzylideneamino)benzenesulfonamide: Synthesis, structure elucidation and their efficiency as PVC photostabilizers. Polymers 2021, 13, 2434. [Google Scholar] [CrossRef]
- Hadi, A.G.; Baqir, S.J.; Ahmed, D.S.; El-Hiti, G.A.; Hashim, H.; Ahmed, A.; Kariuki, B.M.; Yousif, E. Substituted organotin complexes of 4-methoxybenzoic acid for reduction of poly(vinyl chloride) photodegradation. Polymers 2021, 13, 3946. [Google Scholar] [CrossRef]
- Larché, J.F.; Bussière, P.O.; Therias, S.; Gardette, J.L. Photooxidation of polymers: Relating material properties to chemical changes. Polym. Degrad. Stab. 2012, 97, 25–34. [Google Scholar] [CrossRef]
- Scott, G. Mechanism of Polymer Degradation and Stabilization; Elsevier: New York, NY, USA, 1990. [Google Scholar]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; Volume 1, pp. 48–49. [Google Scholar]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohamed, R.R. N-phenyl-3-substituted 5-pyrazolone derivatives as organic stabilizers for rigid poly (vinyl chloride) against photodegradation. J. Appl. Polym. Sci. 2006, 101, 1543–1555. [Google Scholar] [CrossRef]
















| Complex | Melting Point (°C) | Yield (%) | Calculated (Found; %) | |||
|---|---|---|---|---|---|---|
| C | H | N | Sn | |||
| 1 | 105–107 | 83 | 60.03 (60.07) | 4.86 (4.89) | 2.50 (2.52) | 21.19 (21.23) |
| 2 | 174–176 | 74 | 52.82 (52.86) | 7.86 (7.92) | 2.80 (2.84) | 23.73 (23.76) |
| 3 | 123–125 | 84 | 55.43 (55.46) | 9.94 (9.97) | 4.04 (4.06) | 17.12 (17.14) |
| 4 | 108–110 | 81 | 51.47 (51.49) | 6.48 (6.51) | 4.29 (4.33) | 18.17 (18.20) |
| 5 | 178–180 | 78 | 46.42 (46.47) | 5.31 (5.35) | 4.92 (4.93) | 20.86 (20.91) |
| Complex | NH2 | OH | C=O Asym | C=O Sym | Δv (Asym―Sym) | Sn―C | Sn―O |
|---|---|---|---|---|---|---|---|
| 1 | 3450, 3415 | 3271 | 1757 | 1487 | 270 | 511 | 443 |
| 2 | 3473, 3409 | 3217 | 1776 | 1491 | 285 | 530 | 455 |
| 3 | 3452, 3427 | 3277 | 1734 | 1491 | 243 | 522 | 443 |
| 4 | 3444, 3427 | 3252 | 1732 | 1493 | 239 | 524 | 443 |
| 5 | 3431 | 3279 | 1734 | 1497 | 237 | 514 | 457 |
| Complex | 1H NMR (400 MHz) |
|---|---|
| 1 | 8.91 (br s, 1H, OH), 8.79 (br s, 1H, OH), 7.92–7.66 (m, 15H, 3 Ph), 6.60 (s, 1H, Ar), 6.52 (d, J = 7 Hz, 1H, Ar), 6.29 (d, J = 7 Hz, 1H, Ar), 3.45 (br s, 2H, NH2), 2.90 (d, J = 14 Hz, 1H, 1H of CH2), 2.65 (d, J = 14 Hz, 1H, 1H of CH2) 1.28 (s, 3H, Me) |
| 2 | 9.01 (br s, 1H, OH), 7.68 (br s, 1H, OH), 6.67 (d, J = 8 Hz, 1H, Ar), 6.64 (s, 1H, Ar), 6.51 (d, J = 8 Hz, 1H, Ar), 3.81 (br S, 2H, NH2), 2.92 (d, J = 13 Hz, 1H, 1H of CH2), 2.71 (d, J = 13 Hz, 1H, 1H of CH2), 1.64 (br s, 6H, 3 CH2), 1.56–1.48 (m, 12H, 6 CH2), 1.24 (s, 3H, Me), 0.85 (t, J = 7 Hz, 9H, 3 Me) |
| 3 | 9.01 (br s, 2H, 2 OH), 8.90 (br s, 2H, 2 OH), 7.41–7.30 (m, 10H, 2 Ph), 6.68 (s, 2H, Ar), 6.62 (d, J = 8 Hz, 2H, Ar), 6.48 (d, J = 8 Hz, 2H, Ar), 3.73 (br s, 4H, 2 NH2), 2.92 (d, J = 14 Hz, 2H, 1H of CH2), 2.86 (d, J = 14 Hz, 2H, 1H of 2 CH2), 1.23 (s, 6H, 2 Me) |
| 4 | 8.99 (br s, 2H, 2 OH), 8.12 (br s, 2H, 2 OH), 6.68 (d, J = 8 Hz, 2H, Ar), 6.63 (s, 2H, Ar), 6.50 (d, J = 8 Hz, 2H, Ar), 3.65 (br s, 4H, 2 NH2), 2.97 (d, J = 13 Hz, 2H, 1 H of 2 CH2), 2.81 (d, J = 13 Hz, 2H, 1H of 2 CH2), 1.66 (br s, 4H, 2 CH2), 1.51–1.42 (m, 8H, 4 CH2), 1.26 (s, 6H, 2 Me), 0.82 (t, J = 7 Hz, 6H, 2 Me). |
| 5 | 88.88 (br s, 2H, 2 OH), 7.96 (br s, 2H, 2 OH), 6.65 (d, J = 8 Hz, 2H, Ar), 6.53 (s, 2H, Ar), 6.51 (d, J = 8 Hz, 2H, Ar), 3.74 (br s, 4H, 2 NH2), 2.96 (d, J = 14 Hz, 2H, 1 H of 2 CH2), 2.76 (d, J = 14 Hz, 2H, 1H of 2 CH2), 1.40 (s, 6H, 2 Me), 0.51 (s, 6H, 2 Me) |
| Complex | 13C NMR (100 MHz) |
|---|---|
| 1 | 173.1, 145.4, 144.9, 136.7, 128.9, 128.8, 128.4, 125.6, 121.6, 118.6, 116.0, 60.7, 42.5, 23.1 |
| 2 | 173.5, 145.5, 145.0, 126.3, 121.7, 118.6, 116.1, 60.9, 42.6, 28.6, 27.0, 23.1, 20.9, 14.3 |
| 3 | 172.9, 145.6, 145.3, 136.9, 128.8, 128.7, 128.2, 124.8, 121.6, 118.2, 116.1, 60.2, 42.3, 22.3 |
| 4 | 173.0, 145.5, 145.2, 125.0, 121.6, 118.3, 116.1, 60.3, 42.3, 28.0, 26.1, 22.5, 22.2, 14.2 |
| 5 | 174.2, 145.5, 145.1, 125.5, 121.7, 118.5, 116.0, 60.6, 42.4, 23.0, 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naoom, N.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Synthesis of Methyldopa–Tin Complexes and Their Applicability as Photostabilizers for the Protection of Polyvinyl Chloride against Photolysis. Polymers 2022, 14, 4590. https://doi.org/10.3390/polym14214590
Naoom N, Yousif E, Ahmed DS, Kariuki BM, El-Hiti GA. Synthesis of Methyldopa–Tin Complexes and Their Applicability as Photostabilizers for the Protection of Polyvinyl Chloride against Photolysis. Polymers. 2022; 14(21):4590. https://doi.org/10.3390/polym14214590
Chicago/Turabian StyleNaoom, Noor, Emad Yousif, Dina S. Ahmed, Benson M. Kariuki, and Gamal A. El-Hiti. 2022. "Synthesis of Methyldopa–Tin Complexes and Their Applicability as Photostabilizers for the Protection of Polyvinyl Chloride against Photolysis" Polymers 14, no. 21: 4590. https://doi.org/10.3390/polym14214590