Synthesis and Characterization of Schiff Base Polymers via Metal Coordination and Its Application in Infrared Stealth Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Synthesis of Polymers
2.4. Preparation of the Polymer Coatings
3. Results and Discussion
3.1. Synthesis of Polymers and Coordination Polymers
3.2. UV-Vis Absorption Spectroscopic Analysis of Coordination Polymers
3.3. Electrical Conductivity of Polymers and Coordination Polymers
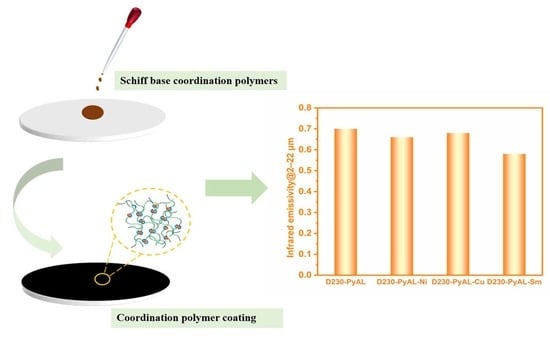
3.4. Infrared Emissivity
3.5. Thermal Properties
3.6. Mechanical Properties
3.7. SEM Micrographs of Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, R.; Xia, X.; Wang, W.; Yu, D. Infrared camouflage fabric prepared by paraffin phase change microcapsule with Good thermal insulting properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124519. [Google Scholar] [CrossRef]
- Kim, D.-G.; Han, K.-I.; Choi, J.-H.; Kim, T.-K. Experimental verification of active IR stealth technology by controlling the surface temperature using a thermoelectric element. J. Mech. Sci. Technol. 2016, 30, 4801–4806. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, S.; Lv, D. Fabrication and characterization of a PDMS modified polyurethane/Al composite coating with super-hydrophobicity and low infrared emissivity. Prog. Org. Coat. 2020, 143, 105622. [Google Scholar] [CrossRef]
- Gu, J.; Wang, W.; Yu, D. Temperature control and low infrared emissivity double-shell phase change microcapsules and their application in infrared stealth fabric. Prog. Org. Coat. 2021, 159, 106439. [Google Scholar] [CrossRef]
- Xiao, L.; Ma, H.; Liu, J.; Zhao, W.; Jia, Y.; Zhao, Q.; Liu, K.; Wu, Y.; Wei, Y.; Fan, S.; et al. Fast Adaptive Thermal Camouflage Based on Flexible VO(2)/Graphene/CNT Thin Films. Nano Lett. 2015, 15, 8365–8370. [Google Scholar] [CrossRef]
- Xu, R.; Wang, W.; Yu, D. A novel multilayer sandwich fabric-based composite material for infrared stealth and super thermal insulation protection. Compos. Struct. 2019, 212, 58–65. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Lou, J.; Zhu, Y.; Gui, B.; Feng, M.; Wang, J.; Qu, S. A thermally robust and optically transparent infrared selective emitter for compatible camouflage. J. Mater. Chem. C 2021, 9, 15018–15025. [Google Scholar] [CrossRef]
- Pan, M.; Huang, Y.; Li, Q.; Luo, H.; Zhu, H.; Kaur, S.; Qiu, M. Multi-band middle-infrared-compatible camouflage with thermal management via simple photonic structures. Nano Energy 2020, 69, 104449. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, Z.; Wu, X.; Li, G.; Fang, D.; Zhang, X. Nanofibrous Kevlar Aerogel Films and Their Phase-Change Composites for Highly Efficient Infrared Stealth. ACS Nano 2019, 13, 2236–2245. [Google Scholar] [CrossRef]
- Liang, J.; Li, W.; Xu, G.; Meng, X.; Liu, K.; Tan, S. Preparation and characterization of the colored coating with low infrared emissivity based on nanometer pigment. Prog. Org. Coat. 2018, 115, 74–78. [Google Scholar] [CrossRef]
- Yu, H.; Xu, G.; Shen, X.; Yan, X.; Shao, C.; Hu, C. Effects of size, shape and floatage of Cu particles on the low infrared emissivity coatings. Prog. Org. Coat. 2009, 66, 161–166. [Google Scholar] [CrossRef]
- Yin, J.; Wang, C.; Zheng, H.; Zhang, L.; Zhang, M.; Ma, X.; Shen, T.; Zhang, W.; Weng, X.; Jiang, S.; et al. Power controlled microstructure and infrared properties of air plasma spraying based on YSZ coatings. Surf. Coat. Technol. 2021, 426, 127768. [Google Scholar] [CrossRef]
- Chai, X.; Zhu, D.; Liu, Y.; Qing, Y.; Ren, Z.; Luo, F.; Zhou, W.; Huang, Z.; Li, P. Silver-modified chromium(III) oxide as multi-band compatible stealth materials for visual/infrared stealth and radar wave transmission. Compos. Sci. Technol. 2021, 216, 109038. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Zhang, T.; He, M.; Bu, X. Fabrication of core–shell structural SiO2@DNA–LDH nanocomposite with low infrared emissivity. Chem. Eng. J. 2015, 266, 199–202. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, M.; Guan, Y.; Huang, X. Laser absorption and infrared stealth properties of Al/ATO composites. Ceram. Int. 2019, 45, 14312–14315. [Google Scholar] [CrossRef]
- Qi, L.; Weng, X.; Wei, B.; Yuan, L.; Huang, G.; Du, X.; Wu, X.; Liu, H. Effects of low-melting glass powder on the thermal stabilities of low infrared emissivity Al/polysiloxane coatings. Prog. Org. Coat. 2020, 142, 105579. [Google Scholar] [CrossRef]
- Wang, K.; Wang, C.; Yin, Y.; Chen, K. Modification of Al pigment with graphene for infrared/visual stealth compatible fabric coating. J. Alloys Compd. 2017, 690, 741–748. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Liu, Y.; Liu, Z.; Ren, Y.; Xue, Y.; Zhu, B.; Wang, R.; Zhang, Q. Preparation of a PCM Microcapsule with a Graphene Oxide Platelet-Patched Shell and Its Thermal Camouflage Applications. Ind. Eng. Chem. Res. 2019, 58, 19090–19099. [Google Scholar] [CrossRef]
- Hafeez, A.; Akhter, Z.; Gallagher, J.F.; Khan, N.A.; Gul, A.; Shah, F.U. Synthesis, Crystal Structures, and Spectroscopic Characterization of Bis-aldehyde Monomers and Their Electrically Conductive Pristine Polyazomethines. Polymers 2019, 11, 1498. [Google Scholar] [CrossRef] [Green Version]
- Nitschke, P.; Jarzabek, B.; Vasylieva, M.; Godzierz, M.; Janeczek, H.; Musioł, M.; Domiński, A. The Effect of Alkyl Substitution of Novel Imines on Their Supramolecular Organization, towards Photovoltaic Applications. Polymers 2021, 13, 1043. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Dou, S.; Zhang, L.; Zhang, H.; Lv, H.; Wang, L.; Zhao, J.; Li, Y. A comprehensive study of electrochromic device with variable infrared emissivity based on polyaniline conducting polymer. Sol. Energy Mater. Sol. Cells 2017, 170, 120–126. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Xu, G.; Wang, L.; Pan, M.; Ren, F.; Chen, X.; Li, X.; Li, Y. Novel aniline and haloaniline binary copolymer films for electro-emissive devices. Mater. Chem. Phys. 2020, 248, 122866. [Google Scholar] [CrossRef]
- Pan, W.; Zhou, Y.; He, M.; Bu, X.; Ding, B.; Huang, T.; Huang, S.; Li, S. Synthesis, helicity, and low infrared emissivity of optically active poly(N-propargylamide)s bearing stigmasteryl moieties. J. Mol. Struct. 2017, 1142, 285–292. [Google Scholar] [CrossRef]
- Louet, C.; Cantin, S.; Dudon, J.-P.; Aubert, P.-H.; Vidal, F.; Chevrot, C. A comprehensive study of infrared reflectivity of poly(3,4-ethylenedioxythiophene) model layers with different morphologies and conductivities. Sol. Energy Mater. Sol. Cells 2015, 143, 141–151. [Google Scholar] [CrossRef]
- Larciprete, M.C.; Paoloni, S.; Orazi, N.; Mercuri, F.; Orth, M.; Gloy, Y.; Centini, M.; Voti, R.L.; Sibilia, C. Infrared emissivity characterization of carbon nanotubes dispersed poly(ethylene terephthalate) fibers. Int. J. Therm. Sci. 2019, 146, 106109. [Google Scholar] [CrossRef]
- Tan, P.; Lu, F.; Han, Y. Mid-infrared optimized electroreflective device based on PEDOT/PSS. Sol. Energy Mater. Sol. Cells 2021, 219, 110808. [Google Scholar] [CrossRef]
- Temizkan, K.; Kaya, İ. Synthesis of soluble poly(azomethine)s containing thiophene and their fluorescence quantum yields. Polym. Bull. 2019, 77, 3287–3303. [Google Scholar] [CrossRef]
- Lei, Z.Q.; Xie, P.; Rong, M.Z.; Zhang, M.Q. Catalyst-free dynamic exchange of aromatic Schiff base bonds and its application to self-healing and remolding of crosslinked polymers. J. Mater. Chem. A 2015, 3, 19662–19668. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Hsieh, T.H.; Huang, Y.C.; Tseng, P.H.; Wang, Y.-Z.; Ho, K.-S.; Huang, Y.-J. Calcined Co(II)-Chelated Polyazomethine as Cathode Catalyst of Anion Exchange Membrane Fuel Cells. Polymers 2022, 14, 1784. [Google Scholar] [CrossRef]
- Croitor, L.; Cocu, M.; Bulhac, I.; Bourosh, P.N.; Kravtsov, V.C.; Petuhov, O.; Danilescu, O. Evolution from discrete mononuclear complexes to trinuclear linear cluster and 2D coordination polymers of Mn(II) with dihydrazone Schiff bases: Preparation, structure and thermal behavior. Polyhedron 2021, 206, 115329. [Google Scholar] [CrossRef]
- Ganguly, R.; Sreenivasulu, B.; Vittal, J.J. Amino acid-containing reduced Schiff bases as the building blocks for metallasupramolecular structures. Coord. Chem. Rev. 2008, 252, 1027–1050. [Google Scholar] [CrossRef]
- Kitaura, R.; Onoyama, G.; Sakamoto, H.; Matsuda, R.; Noro, S.-I.; Kitagawa, S. Immobilization of a metallo schiff base into a microporous coordination polymer. Angew. Chem. Int. Ed. 2004, 43, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Han, G.C.; Chen, S.L.; Chen, Z. Inhibition of copper corrosion by the formation of Schiff base self-assembled monolayers. Appl. Surf. Sci. 2016, 389, 601–608. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Sayed, A.R.; Shalabi, K. Synthesis and theoretical studies of novel conjugated polyazomethines and their application as efficient inhibitors for C1018 steel pickling corrosion behavior. Surf. Interfaces 2021, 23, 101037. [Google Scholar] [CrossRef]
- Halevi, O.; Chen, J.; Thangavel, G.; Morris, S.A.; Ben Uliel, T.; Tischler, Y.R.; Lee, P.S.; Magdassi, S. Synthesis through 3D printing: Formation of 3D coordination polymers. RSC Adv. 2020, 10, 14812–14817. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Ray, S. Zeolite encapsulated Ni(II) Schiff-base complexes: Improved catalysis and site isolation. New J. Chem. 2020, 44, 14953–14963. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Z.-X.; He, X.; Shao, M.; Li, M.-X. One unexpected mixed-valence Cu(I,II)-cyanide coordination polymer in situ originating from the cleavage of acetonitrile. Inorg. Chem. Commun. 2017, 80, 46–48. [Google Scholar] [CrossRef]
- Li, J.; Lv, F.; Yang, R.; Zhang, L.; Tao, W.; Liu, G.; Gao, H.; Guan, Y. N-Doped Biochar from Lignocellulosic Biomass for Preparation of Adsorbent: Characterization, Kinetics and Application. Polymers 2022, 14, 3889. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Han, J.; Ma, G.; Liu, X.; Wang, J.; Jian, X. Construction of dimetal-containing dithiolene and Schiff base conjugated polymer coating: Exploiting metal coordination as a design strategy for improving infrared stealth properties. Polym. Chem. 2019, 10, 5839–5848. [Google Scholar] [CrossRef]
- Tian, P.; Cheng, J.; Zhang, G. X-ray photoelectron spectroscopy of Sm3+-doped CaO–MgO–Al2O3–SiO2 glasses and glass ceramics. Appl. Surf. Sci. 2011, 257, 4896–4900. [Google Scholar] [CrossRef]
- Gottfried, J.M.; Flechtner, K.; Kretschmann, A.C.; Lukasczyk, T.; Steinrück, H.P. Direct synthesis of a metalloporphyrin complex on a surface. J. Am. Chem. Soc. 2006, 128, 5644–5645. [Google Scholar] [CrossRef]
- Morsi, R.M.M.; Mandour, H.S.; Fathi, A.M.; Awad, H.M. Electrical properties, cyclic voltammetry, and anticancer activities of N-(4-(2-hydrazinyl-2-oxoethoxy)phenyl) acetamide complexes. J. Phys. Org. Chem. 2019, 32, e3945. [Google Scholar] [CrossRef]
- Yin, H.; Tang, Z.; Fu, G.; Ai, X.; Xia, J.; Tang, H.; Yang, C.; Qu, L.; Li, Y. Novel rare earth coordination polymers with greatly enhanced fluorescence by synergistic effect of carboxyl-functionalized poly(arylene ether nitrile) and 1,10-phenanthroline. Eur. Polym. J. 2020, 141, 110078. [Google Scholar] [CrossRef]
- Olgun, U.; Gülfen, M. Doping of poly(o-phenylenediamine): Spectroscopy, voltammetry, conductivity and band gap energy. React. Funct. Polym. 2014, 77, 23–29. [Google Scholar] [CrossRef]
- Wang, B.-H.; Xi, H.-A.; Yin, J.; Qian, X.-F.; Zhu, Z.-K. Molecular orbital confinement effect of mesoporous silica of MCM-41 on conjugated polymer. Synth. Met. 2003, 139, 187–190. [Google Scholar] [CrossRef]
- Duru Kamaci, U.; Kamaci, M.; Peksel, A. A dual responsive colorimetric sensor based on polyazomethine and ascorbic acid for the detection of Al (III) and Fe (II) ions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 254, 119650. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.M.; Anziliero, S.; Camassola, M.; Coulon Grisa, A.M.; Brandalise, R.N.; Zeni, M. Evaluation of Metal Biosorption by the Fungus Pleurotus sajor-caju on Modified Polyethylene Films. J. Bioremediat. Biodegrad. 2012, 3, 152. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Wang, L.; Li, L.; Feng, S. Imine-functionalized polysiloxanes for supramolecular elastomers with tunable mechanical properties. Polym. Chem. 2020, 11, 7721–7728. [Google Scholar] [CrossRef]










| Polymer Name | λonset | Eg |
|---|---|---|
| (nm) | (eV) | |
| D230-PyAL | 359 | 3.45 |
| D230-PyAL-Ni | 374 | 3.32 |
| D230-PyAL-Cu | 365 | 3.40 |
| D230-PyAL-Sm | 414 | 2.99 |
| Polymer Name | Resistivity | Conductivity |
|---|---|---|
| (Ω cm) | (×10−4 S/cm) | |
| D230-PyAL | 7508 | 1.35 |
| D230-PyAL-Ni | 3443 | 2.94 |
| D230-PyAL-Cu | 4029 | 2.55 |
| D230-PyAL-Sm | 2873 | 3.54 |
| Polymer Name | ε2–22μm |
|---|---|
| D230-PyAL | 0.70 |
| D230-PyAL-Ni | 0.66 |
| D230-PyAL-Cu | 0.68 |
| D230-PyAL-Sm | 0.58 |
| Polymer Name | Td5% | Td10% | Char Yield |
|---|---|---|---|
| D230-PyAL | 262 | 278 | 30 |
| D230-PyAL-Ni | 237 | 258 | 52 |
| D230-PyAL-Cu | 264 | 291 | 51 |
| D230-PyAL-Sm | 271 | 297 | 55 |
| Polymer Name | E | H | H/E |
|---|---|---|---|
| (GPa) | (GPa) | ||
| D230-PyAL | 2.498 ± 0.001 | 0.042 ± 0.001 | 0.016 |
| D230-PyAL-Ni | 4.343 ± 0.002 | 0.203 ± 0.001 | 0.047 |
| D230-PyAL-Cu | 4.483 ± 0.001 | 0.230 ± 0.001 | 0.051 |
| D230-PyAL-Sm | 4.175 ± 0.003 | 0.195 ± 0.001 | 0.047 |
| Polymer Name | Impact Resistance a | Flexibility b | Adhesive Force |
|---|---|---|---|
| (cm) | (mm) | (Grade) | |
| D230-PyAL | 50 | 0.5 | 0 |
| D230-PyAL-Ni | 50 | 1 | 1 |
| D230-PyAL-Cu | 10 | 2.5 | 1 |
| D230-PyAL-Sm | 50 | 0.5 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zong, L.; Li, W.; Wang, Y.; Wang, J.; Jian, X. Synthesis and Characterization of Schiff Base Polymers via Metal Coordination and Its Application in Infrared Stealth Coating. Polymers 2022, 14, 4563. https://doi.org/10.3390/polym14214563
Li X, Zong L, Li W, Wang Y, Wang J, Jian X. Synthesis and Characterization of Schiff Base Polymers via Metal Coordination and Its Application in Infrared Stealth Coating. Polymers. 2022; 14(21):4563. https://doi.org/10.3390/polym14214563
Chicago/Turabian StyleLi, Xiangyu, Lishuai Zong, Weijie Li, Yibo Wang, Jinyan Wang, and Xigao Jian. 2022. "Synthesis and Characterization of Schiff Base Polymers via Metal Coordination and Its Application in Infrared Stealth Coating" Polymers 14, no. 21: 4563. https://doi.org/10.3390/polym14214563