The Frontiers of Functionalized Nanocellulose-Based Composites and Their Application as Chemical Sensors
Abstract
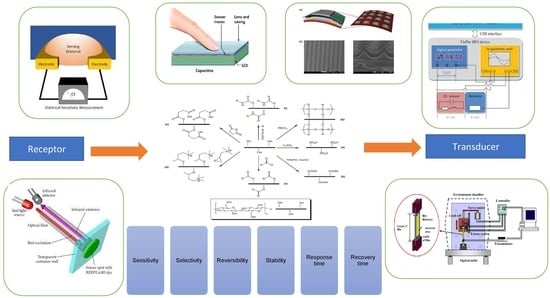
:1. Introduction
2. An Introduction to Chemical Sensors
3. Nanocellulose’s Unique Characteristics as a Chemical Sensor
4. Chemical Sensor Characteristics and Their Basic Mechanism
5. Performance of Nanocellulose Composite Chemical Sensors
6. Performance of Nanocellulose Hybrid Composite Chemical Sensors
7. Challenges and Future Directions
- (a)
- Agglomeration is a major issue that must be taken into account when preparing nanocellulose for use as this is a result of nanocellulose’s inherent large surface area properties. It should be emphasized that aggregated nanocellulose is very difficult to be re-dispersed, which can lead to undesirable effects on sensing abilities and mechanical properties of the resultant composites. Due to its hydrophilic properties, nanocellulose is also difficult to dry, becomes prone to aggregation and is incompatible with other hydrophobic materials.
- (b)
- Functionalization is an important key towards success in order to make nanocellulose a conducting polymer composite. There is still gaps in knowledge of the crystallinity and surface properties of functionalized nanocellulose, which can have a significant impact on the fabrication, reproducibility and applications of nanocellulose composites within chemical sensors. Methods such as nuclear magnetic resonance spectroscopy can be used to determine the chemical structure of these materials. This functionalization has been a key factor in many of the sensor technologies described in this review. Many of the sensor technologies highlighted have shown high sensitivity and selectivity and low cost, which are essential attributes for their commercialization and wider use in the industry.
- (c)
- Pretreatment has been a feature in a number of the developments described earlier. The development of compact and portable nanocellulose composite chemical sensors which require less complicated pretreatment steps is a matter that needs attention to capitalise on the advantages that nanocellulose composites possess to enable better and more efficient sensors to be made.
- (d)
- Major hurdles for the commercial application of nanocellulose lies both in their processes of fabrication and post-production preparation. Green nanocellulose extraction approaches and functionalization techniques that minimize acid and solvent wastes are needed. Moreover, the presence of chemical contaminants arising from functionalization processes must be avoided
- (e)
- Research aimed at optimizing the dispersion and orientation of nanocellulose within or on composite matrices needs further exploration. This is an important key towards structural integrity and sensing performance of these sensors. There have been several studies exploring the use of a variety of different compatibilizer in polymer composites of nanocellulose aimed at improving the dispersion and arrangement of fibers in these composites.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilyas, R.A.; Nurazzi, N.M.; Norrrahim, M.N.F. Fiber-Reinforced Polymer Nanocomposites. Nanomaterials 2022, 12, 3045. [Google Scholar] [CrossRef] [PubMed]
- Asyraf, M.R.M.; Ishak, M.R.; Syamsir, A.; Nurazzi, N.M.; Sabaruddin, F.A.; Shazleen, S.S.; Norrrahim, M.N.F.; Rafidah, M.; Ilyas, R.A.; Abd Rashid, M.Z.; et al. Mechanical Properties of Oil Palm Fibre-Reinforced Polymer Composites: A Review. J. Mater. Res. Technol. 2021, 17, 33–65. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, M.S.; Norizan, M.N.; Norrrahim, M.N.F.; Ibrahim, R.; Atikah, M.S.N.; Huzaifah, M.R.M.; Radzi, A.M.; Izwan, S.; Azammi, A.M.N.; et al. Macro to Nanoscale Natural Fiber Composites for Automotive Components: Research, Development, and Application. In Biocomposite and Synthetic Composites for Automotive Applications; Sapuan, M.S., Ilyas, R.A., Eds.; Woodhead Publishing Series: Amsterdam, The Netherland, 2020. [Google Scholar]
- Asyraf, M.R.M.; Ishak, M.R.; Norrrahim, M.N.F.; Nurazzi, N.M.; Shazleen, S.S.; Ilyas, R.A.; Rafidah, M.; Razman, M.R. Recent Advances of Thermal Properties of Sugar Palm Lignocellulosic Fibre Reinforced Polymer Composites. Int. J Biol. Macromol. 2021, 193, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, S.H.; Padzil, F.N.M.; Ainun, Z.M.A.; Norrrahim, M.N.F.; Chin, K.L. Biocomposites and Nanocomposites. In Composite Materials; CRC Press: Boca Raton, FL, USA, 2021; pp. 29–60. [Google Scholar]
- Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Sapuan, S.M.; Ilyas, R.A.; Hakimi, M.I.; Najmuddin, S.U.F.S.; Jenol, M.A. Nanocellulose Reinforced Polypropylene and Polyethylene Composite for Packaging Application. In Bio-Based Packaging: Material, Environmental and Economic Aspects; Wiley Online Library: Hoboken, NJ, USA, 2021. [Google Scholar]
- Norizan, M.N.; Alias, A.H.; Sabaruddin, F.A.; Asyraf, M.R.M.; Shazleen, S.S.; Mohidem, N.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Rushdan, A.I.; Ishak, M.R.; et al. Effect of Silane Treatments on Mechanical Performance of Kenaf Fibre Reinforced Polymer Composites: A Review. Funct. Compos. Struct. 2021, 3, 045003. [Google Scholar]
- Nurazzi, N.M.; Norrrahim, M.N.F.; Sabaruddin, F.A.; Shazleen, S.S.; Ilyas, R.A.; Lee, S.H.; Padzil, F.N.M.; Aizat, G.; Aisyah, H.A.; Mohidem, N.A.; et al. Mechanical Performance Evaluation of Bamboo Fibre Reinforced Polymer Composites and Its Applications: A Review. Funct. Compos. Struct. 2022, 4, 015009. [Google Scholar] [CrossRef]
- Nasri, A.; Pétrissans, M.; Fierro, V.; Celzard, A. Gas Sensing Based on Organic Composite Materials: Review of Sensor Types, Progresses and Challenges. Mater. Sci. Semicond. Process. 2021, 128, 105744. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Abdullah, N.; Demon, S.Z.N.; Halim, N.A.; Azmi, A.F.M.; Knight, V.F.; Mohamad, I.S. The Frontiers of Functionalized Graphene-Based Nanocomposites as Chemical Sensors. Nanotechnol. Rev. 2021, 10, 330–369. [Google Scholar] [CrossRef]
- Yang, Y.; Tu, H.; Zhang, A.; Du, D.; Lin, Y. Preparation and Characterization of Au-ZrO2-SiO2 Nanocomposite Spheres and Their Application in Enrichment and Detection of Organophosphorus Agents. J. Mater. Chem. 2012, 22, 4977–4981. [Google Scholar] [CrossRef]
- Misenan, M.S.M.; Janudin, N.; Idayu, M.A.; Norrrahim, M.N.F.; Jamal, S.H.; Wan Yusoff, W.Y.; Kasim, N.; Yunus, W.M.D.Z.W.; Ernest, V.F.K.V.; Kasim, N.A.M. Cellulose Nanofiber as Potential Absorbent Material for Chloride Ion. Solid State Phenom. 2021, 317, 263–269. [Google Scholar] [CrossRef]
- Janudin, N.; Kasim, N.A.M.; Knight, V.F.; Halim, N.A.; Noor, S.A.M.; Ong, K.K.; Yunus, W.M.Z.W.; Norrrahim, M.N.F.; Misenan, M.S.M.; Razak, M.A.I.A.; et al. Sensing Techniques on Determination of Chlorine Gas and Free Chlorine in Water. J. Sens. 2022, 2022, 1898417. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ujang, F.A.; Janudin, N.; Razak, M.A.I.A.; Shah, N.A.A.; Noor, S.A.M.; Jamal, S.H.; Ong, K.K.; et al. Nanocellulose: The Next Super Versatile Material for the Military. Mater. Adv. 2021, 2, 1485–1506. [Google Scholar] [CrossRef]
- Hemath, M.; Mavinkere Rangappa, S.; Kushvaha, V.; Dhakal, H.N.; Siengchin, S. A Comprehensive Review on Mechanical, Electromagnetic Radiation Shielding, and Thermal Conductivity of Fibers/Inorganic Fillers Reinforced Hybrid Polymer Composites. Polym. Compos. 2020, 41, 3940–3965. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha-Silva, H.; Julia Arcos-Martinez, M. Development of a Selective Chloride Sensing Platform Using a Screen-Printed Platinum Electrode. Talanta 2019, 195, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Jaggi, N.; Nathawat, R. Functionalized Multiwalled Carbon Nanotubes Based Hydrogen Gas Sensor. Sens. Actuators A Phys. 2013, 201, 321–327. [Google Scholar] [CrossRef]
- Janudin, N.; Abdullah, N.; Wan Yunus, W.M.Z.; Yasin, F.M.; Yaacob, M.H.; Mohamad Saidi, N.; Kasim, N.A.M. Effect of Functionalized Carbon Nanotubes in the Detection of Benzene at Room Temperature. J. Nanotechnol. 2018, 2018, 2107898. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Mahajan, A.; Saini, R.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Reversible and Fast Responding Ppb Level Cl2 Sensor Based on Noncovalent Modified Carbon Nanotubes with Hexadecafluorinated Copper Phthalocyanine. Sens. Actuators B Chem. 2018, 255, 87–99. [Google Scholar] [CrossRef]
- Fennell, J.; Hamaguchi, H.; Yoon, B.; Swager, T. Chemiresistor Devices for Chemical Warfare Agent Detection Based on Polymer Wrapped Single-Walled Carbon Nanotubes. Sensors 2017, 17, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janudin, N.; Kasim, N.A.M.; Feizal Knight, V.; Norrrahim, M.N.F.; Razak, M.A.I.A.; Abdul Halim, N.; Mohd Noor, S.A.; Ong, K.K.; Yaacob, M.H.; Ahmad, M.Z.; et al. Fabrication of a Nickel Ferrite/Nanocellulose-Based Nanocomposite as an Active Sensing Material for the Detection of Chlorine Gas. Polymers 2022, 14, 1906. [Google Scholar] [CrossRef] [PubMed]
- Nurazzi, N.M.; Demon, S.Z.N.; Halim, N.A.; Mohamad, I.S.; Abdullah, N. Carbon Nanotubes-Based Sensor for Ammonia Gas Detection—An Overview. Polimery 2021, 66, 175–186. [Google Scholar] [CrossRef]
- Gao, J.; He, S.; Nag, A. Electrochemical Detection of Glucose Molecules Using Laser-Induced Graphene Sensors: A Review. Sensors 2021, 21, 2818. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Atikah, M.S.N.; Atiqah, A.; Ansari, M.N.M.; Norrrahim, M.N.F. Production, Processes and Modification of Nanocrystalline Cellulose from Agro-Waste: A Review. In Nanocrystalline Materials; IntechOpen: London, UK, 2020; pp. 1–30. [Google Scholar]
- Mohd Nurazzi, N.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, C.; Curto, V.F.; Matzeu, G.; Fraser, K.J.; Diamond, D. Properties and Customization of Sensor Materials for Biomedical Applications. Compr. Mater. Process. 2014, 13, 221–243. [Google Scholar] [CrossRef] [Green Version]
- Thiruganasambanthan, T.; Ilyas, R.A.; Norrrahim, M.N.F.; Kumar, T.S.M.; Siengchin, S.; Misenan, M.S.M.; Farid, M.A.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Zakaria, S.Z.S.; et al. Emerging Developments on Nanocellulose as Liquid Crystals: A Biomimetic Approach. Polymers 2022, 14, 1546. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ong, K.K.; Noor, S.A.M.; Jamal, S.H.; Shah, N.A.A.; Halim, N.A.; Ilyas, R.A.; Yunus, W.M.Z.W. Cationic Nanocellulose as Promising Candidate for Filtration Material of COVID-19: A Perspective. Appl. Sci. Eng. Prog. 2021, 14, 580–587. [Google Scholar] [CrossRef]
- Shukor, N.N.; Roslan, N.F.; Nurazzi, N.M.; Shazleen, S.S.; Faiz Norrrahim, M.N.; Knight, V.F.; Mohamad Nor, N. An Overview on Chemical Contaminants of Wastewater and Their Current Removal Techniques. Asian J. Water Environ. Pollut. 2022, 19, 15–22. [Google Scholar] [CrossRef]
- Ghosh, S.; Maiyalagan, T.; Basu, R.N. Nanostructured Conducting Polymers for Energy Applications: Towards a Sustainable Platform. Nanoscale 2016, 8, 6921–6947. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials Enhanced Surface Plasmon Resonance for Biological and Chemical Sensing Applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Biju, V. Chemical Modifications and Bioconjugate Reactions of Nanomaterials for Sensing, Imaging, Drug Delivery and Therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, G.; Nawaz, A.; Nawaz, S.; Shad, N.A.; Sajid, M.M.; Javed, Y. Nanomaterial-Based Gas Sensor for Environmental Science and Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128207024. [Google Scholar]
- Chen, Z.; Wang, J.; Wang, Y. Strategies for the performance enhancement of graphene-based gas sensors: A review. Talanta 2021, 235, 122745. [Google Scholar] [CrossRef]
- Swager, T.M.; Mirica, K.A. Introduction: Chemical Sensors. Chem. Rev. 2019, 119, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Rzaij, J.M.; Abass, A.M. Review on: TiO2 Thin Film as a Metal Oxide Gas Sensor. J. Chem. Rev. 2020, 2, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Ponzoni, A.; Baratto, C.; Cattabiani, N.; Falasconi, M.; Galstyan, V.; Nunez-Carmona, E.; Rigoni, F.; Sberveglieri, V.; Zambotti, G.; Zappa, D. Smetal Oxide Gas Sensors, a Survey of Selectivity Issues Addressed at the SENSOR Lab, Brescia (Italy). Sensors 2017, 17, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demon, S.Z.N.; Kamisan, A.I.; Abdullah, N.; Noor, S.A.M.; Khim, O.K.; Kasim, N.A.M.; Yahya, M.Z.A.; Manaf, N.A.A.; Azmi, A.F.M.; Halim, N.A. Graphene-Based Materials in Gas Sensor Applications: A Review. Sens. Mater. 2020, 32, 759–777. [Google Scholar] [CrossRef] [Green Version]
- Nurazzi, N.M.; Harussani, M.M.; Siti Zulaikha, N.D.; Norhana, A.H.; Imran Syakir, M.; Norli, A. Composites Based on Conductive Polymer with Carbon Nanotubes in DMMP Gas Sensors—An Overview. Polimery 2021, 66, 85–97. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon Nanotubes: Functionalisation and Their Application in Chemical Sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Naficy, S.; Chandrawati, R.; Dehghani, F. Nanocellulose for Sensing Applications. Adv. Mater. Interfaces 2019, 6, 30–33. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The application status of nanoscale cellulose-based hydrogels in tissue engineering and regenerative biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 732513. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yu, H.; Zhang, Y.; Dong, F.; Li, Z. Colloids and Surfaces B: Biointerfaces Cellulose Acetate Nanofibers Coated Layer-by-Layer with Polyethylenimine and Graphene Oxide on a Quartz Crystal Microbalance for Use as a Highly Sensitive Ammonia Sensor. Colloids Surf. B Biointerfaces 2016, 148, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Kumar, S.; Ngasainao, M.R.; Sharma, D.; Sengar, M.; Gahlot, A.P.S.; Shukla, S.; Kumari, P. Contemporary Nanocellulose-Composites: A New Paradigm for Sensing Applications. Carbohydr. Polym. 2022, 298, 120052. [Google Scholar] [CrossRef]
- Misenan, M.S.M.; Akhlisah, Z.N.; Shaffie, A.H.; Saad, M.A.M.; Norrrahim, M.N.F. 8—Nanocellulose in Sensors. In Woodhead Publishing Series in Composites Science and Engineering; Sapuan, S.M., Norrrahim, M.N.F., Ilyas, R.A., Soutis, C.B.T.-I.A., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 213–243. ISBN 978-0-323-89909-3. [Google Scholar]
- Gabrielli, V.; Frasconi, M. Cellulose-Based Functional Materials for Sensing. Chemosensors 2022, 10, 352. [Google Scholar] [CrossRef]
- Divya; Mahapatra, S.; Srivastava, V.R.; Chandra, P. Nanobioengineered Sensing Technologies Based on Cellulose Matrices for Detection of Small Molecules, Macromolecules, and Cells. Biosensors 2021, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, K.B.R.; Sanfelice, R.C.; Migliorini, F.L.; Pavinatto, A.; Facure, M.H.M.; Correa, D.S. A Review on the Role and Performance of Cellulose Nanomaterials in Sensors. ACS Sens. 2021, 6, 2473–2496. [Google Scholar] [CrossRef] [PubMed]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef] [PubMed]
- Mbakop, S.; Nthunya, L.N.; Onyango, M.S. Recent advances in the synthesis of nanocellulose functionalized–hybrid membranes and application in water quality improvement. Processes 2021, 9, 611. [Google Scholar] [CrossRef]
- Ariffin, H.; Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Nishida, H.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W. Oil Palm Biomass Cellulose-Fabricated Polylactic Acid Composites for Packaging Applications. In Bionanocomposites for Packaging Applications; Springer: Cham, Switzerland, 2018; pp. 95–105. ISBN 9783319673196. [Google Scholar]
- Lawal, A.A.; Hassan, M.A.; Zakaria, M.R.; Yusoff, M.Z.M.; Norrrahim, M.N.F.; Mokhtar, M.N.; Shirai, Y. Effect of Oil Palm Biomass Cellulosic Content on Nanopore Structure and Adsorption Capacity of Biochar. Bioresour. Technol. 2021, 332, 125070. [Google Scholar] [CrossRef]
- Faiz Norrrahim, M.N.; Ahmad Farid, M.A.; Lawal, A.A.; Tengku Yasim-Anuar, T.A.; Samsudin, M.H.; Zulkifli, A.A. Emerging Technologies for Value-Added Use of Oil Palm Biomass. Environ. Sci. Adv. 2022, 1, 259–275. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ariffin, H.; Yasim-Anuar, T.A.T.; Hassan, M.A.; Nishida, H.; Tsukegi, T. One-Pot Nanofibrillation of Cellulose and Nanocomposite Production in a Twin-Screw Extruder. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012034. [Google Scholar] [CrossRef]
- Sharip, N.S.; Yasim-Anuar, T.A.T.; Norrrahim, M.N.F.; Shazleen, S.S.; Nurazzi, N.M.; Sapuan, S.M.; Ilyas, R.A. A Review on Nanocellulose Composites in Biomedical Application. In Composites in Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 161–190. [Google Scholar]
- Norrrahim, M.N.F.; Ariffin, H.; Yasim-Anuar, T.A.T.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Nishida, H. Performance Evaluation of Cellulose Nanofiber with Residual Hemicellulose as a Nanofiller in Polypropylene-Based Nanocomposite. Polymers 2021, 13, 1064. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Ariffin, H.; Hassan, M.A.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Nishida, H. Utilisation of Superheated Steam in Oil Palm Biomass Pretreatment Process for Reduced Chemical Use and Enhanced Cellulose Nanofibre Production. Int. J. Nanotechnol. 2019, 16, 668–679. [Google Scholar] [CrossRef]
- Ariffin, H.; Tengku Yasim-Anuar, T.A.; Norrrahim, M.N.F.; Hassan, M.A. Synthesis of Cellulose Nanofiber from Oil Palm Biomass by High Pressure Homogenization and Wet Disk Milling. In Nanocellulose: Synthesis, Structure, Properties and Applications; World Scientific: Singapore, 2021; pp. 51–64. [Google Scholar]
- Norrrahim, M.N.F.; Ariffin, H.; Yasim-Anuar, T.A.T.; Ghaemi, F.; Hassan, M.A.; Ibrahim, N.A.; Ngee, J.L.H.; Yunus, W.M.Z.W. Superheated Steam Pretreatment of Cellulose Affects Its Electrospinnability for Microfibrillated Cellulose Production. Cellulose 2018, 25, 3853–3859. [Google Scholar] [CrossRef]
- Nor, M.; Norrrahim, F.; Azilah, N.; Kasim, M.; Knight, V.F.; Janudin, N.; Arisyah, T.; Yasim-Anuar, T.; Halim, N.A.; Aisyah, N.; et al. Mini review on nanofibrillation techniques to obtain cellulose nanofiber from lignocellulosic biomass article info abstract. Zulfaqar. J. Def. Mgt. Soc. Sci. Hum. 2021, 4, 134–145. [Google Scholar]
- Norrrahim, M.N.F.; Huzaifah, M.R.M.; Farid, M.A.A.; Shazleen, S.S.; Misenan, M.S.M.; Yasim-Anuar, T.A.T.; Naveen, J.; Nurazzi, N.M.; Rani, M.S.A.; Hakimi, M.I.; et al. Greener Pretreatment Approaches for the Valorisation of Natural Fibre Biomass into Bioproducts. Polymers 2021, 13, 2971. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Tortorella, S.; Buratti, V.V.; Maturi, M.; Sambri, L.; Franchini, M.C.; Locatelli, E. Surface-Modified Nanocellulose for Application in Biomedical Engineering and Nanomedicine: A Review. Int. J. Nanomed. 2020, 15, 9909. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in Sensing and Biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose Properties and Applications in Colloids and Interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Schyrr, B.; Pasche, S.; Voirin, G.; Weder, C.; Simon, Y.C.; Foster, E.J. Biosensors Based on Porous Cellulose Nanocrystal–Poly (Vinyl Alcohol) Scaffolds. ACS Appl. Mater. Interfaces 2014, 6, 12674–12683. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Guleria, A.; Kumar, S.; Sharma, S.; Singh, K. Recent advances in nanocellulose processing, functionalization and applications: A review. Mater. Adv. 2021, 2, 1872–1895. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Misenan, M.S.M.; Janudin, N.; Shah, N.A.A.; Kasim, N.; Yusoff, W.Y.W.; Noor, S.A.M.; Jamal, S.H.; et al. Nanocellulose: A Bioadsorbent for Chemical Contaminant Remediation. RSC Adv. 2021, 11, 7347–7368. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, J.; Liu, S.; Zou, M.; Lu, S.; Yang, Y.; Liao, C.; Wang, Y. A High-Strength Strain Sensor Based on a Reshaped Micro-Air-Cavity. Sensors 2020, 20, 4530. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.M.; Abdullah, L.C.; Tahir, P.M.; Zaini, L.H. Cellulosic Nanomaterials for Sensing Applications. In Handbook of Green Materials: 3 Self-and Direct-Assembling of Bionanomaterials; World Scientific: Singapore, 2014; pp. 197–212. [Google Scholar]
- Wei, X.; Lin, T.; Duan, M.; Du, H.; Yin, X. Cellulose Nanocrystal-Based Liquid Crystal Structures and the Unique Optical Characteristics of Cellulose Nanocrystal Films. Bioresources 2021, 16, 2110–2137. [Google Scholar] [CrossRef]
- Koval, V.; Barbash, V.; Dusheyko, M.; Lapshuda, V.; Yashchenko, O.; Yakimenko, Y. Application of Nanocellulose in Humidity Sensors for Biodegradable Electronics. In Proceedings of the 2020 IEEE 10th International Conference Nanomaterials: Applications & Properties (NAP), Sumy, Ukraine, 9–13 November 2020. [Google Scholar]
- Norrrahim, M.N.F.; Mohd Kasim, N.A.; Knight, V.F.; Ong, K.K.; Mohd Noor, S.A.; Abdul Halim, N.; Ahmad Shah, N.A.; Jamal, S.H.; Janudin, N.; Misenan, M.S.M.; et al. Emerging Developments Regarding Nanocellulose-Based Membrane Filtration Material against Microbes. Polymers 2021, 13, 3249. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Norizan, M.N.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.T.; Najmuddin, S.U.F.S.; Ilyas, R.A. Emerging Development on Nanocellulose as Antimicrobial Material: An Overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.; Luong, J.H.T. Applications of Functionalized and Nanoparticle-Modified Nanocrystalline Cellulose. Trends Biotechnol. 2012, 20, 283–290. [Google Scholar] [CrossRef]
- Misenan, S.; Shaffie, A.; Zulkipli, N.; Norrrahim, F. Nanocellulose in Sensors. In Industrial Applications of Nanocellulose and Its Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 213–240. ISBN 9780323899093. [Google Scholar]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Saalah, S.; Lenggoro, W. Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes 2022, 12, 287. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Alrammouz, R.; Podlecki, J.; Abboud, P.; Sorli, B.; Habchi, R. A Review on Flexible Gas Sensors: From Materials to Devices. Sens. Actuators A Phys. 2018, 284, 209–231. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive Polyaniline-Based Gas Sensors: A Mini Review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Leelaarporn, P.; Wachiraphan, P.; Kaewlee, T.; Udsa, T.; Chaisaen, R.; Choksatchawathi, T.; Laosirirat, R.; Lakhan, P.; Natnithikarat, P.; Thanontip, K.; et al. Sensor-driven achieving of smart living: A review. IEEE Sens. J. 2021, 21, 10369–10391. [Google Scholar] [CrossRef]
- He, S.; Yuan, Y.; Nag, A.; Feng, S.; Afsarimanesh, N.; Han, T.; Mukhopadhyay, S.C.; Organ, D.R. A Review on the Use of Impedimetric Sensors for the Inspection of Food Quality. Int. J. Environ. Res. Public Health 2020, 17, 5220. [Google Scholar] [CrossRef]
- Lowe, B.M.; Sun, K.; Zeimpekis, I.; Skylaris, C.K.; Green, N.G. Field-Effect Sensors-from PH Sensing to Biosensing: Sensitivity Enhancement Using Streptavidin-Biotin as a Model System. Analyst 2017, 142, 4173–4200. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Wu, M.; Hong, Y.; Jeong, Y.; Jung, G.; Shin, W.; Park, J.; Kim, D.; Jang, D.; Lee, J.H. FET-Type Gas Sensors: A Review. Sens. Actuators B Chem. 2021, 330, 129240. [Google Scholar] [CrossRef]
- Saga, S. Mechanical Sensors. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-801238-3. [Google Scholar]
- Xu, K.; Ha, N.; Hu, Y.; Ma, Q.; Chen, W.; Wen, X.; Ou, R.; Trinh, V.; McConville, C.F.; Zhang, B.Y.; et al. A Room Temperature All-Optical Sensor Based on Two-Dimensional SnS2 for Highly Sensitive and Reversible NO2 Sensing. J. Hazard. Mater. 2021, 426, 127813. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Dang, H.; Meng, F. Structure Design and Application of Hollow Core Microstructured Optical Fiber Gas Sensor: A Review. Opt. Laser Technol. 2021, 135, 106658. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional Nanomaterials through Esterification of Cellulose: A Review of Chemistry and Application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef] [Green Version]
- Ye, G.; Zhu, X.; Chen, S.; Li, D.; Yin, Y.; Lu, Y.; Komarneni, S.; Yang, D. Nanoscale Engineering of Nitrogen-Doped Carbon Nanofiber Aerogels for Enhanced Lithium Ion Storage. J. Mater. Chem. A Mater. 2017, 5, 8247–8254. [Google Scholar] [CrossRef]
- Rafieian, F.; Hosseini, M.; Jonoobi, M.; Yu, Q. Development of Hydrophobic Nanocellulose-Based Aerogel via Chemical Vapor Deposition for Oil Separation for Water Treatment. Cellulose 2018, 25, 4695–4710. [Google Scholar] [CrossRef]
- Han, T.; Nag, A.; Chandra Mukhopadhyay, S.; Xu, Y. Carbon Nanotubes and Its Gas-Sensing Applications: A Review. Sens. Actuators A Phys. 2019, 291, 107–143. [Google Scholar] [CrossRef]
- Kale, G.M.; Jafferson, J.M. Solid-State Gas Sensors: Design and Fabrication; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780128035818. [Google Scholar]
- Wang, B.; Dai, L.; Hunter, L.A.; Zhang, L.; Yang, G.; Chen, J.; Zhang, X.; He, Z.; Ni, Y. A Multifunctional Nanocellulose-Based Hydrogel for Strain Sensing and Self-Powering Applications. Carbohydr. Polym. 2021, 268, 118210. [Google Scholar] [CrossRef]
- Hanif, Z.; Shin, D.; Choi, D.; Park, S.J. Development of a Vapor Phase Polymerization Method Using a Wet-on-Wet Process to Coat Polypyrrole on Never-Dried Nanocellulose Crystals for Fabrication of Compression Strain Sensor. Chem. Eng. J. 2020, 381, 122700. [Google Scholar] [CrossRef]
- Ouyang, Z.; Xu, D.; Yu, H.Y.; Li, S.; Song, Y.; Tam, K.C. Novel Ultrasonic-Coating Technology to Design Robust, Highly Sensitive and Wearable Textile Sensors with Conductive Nanocelluloses. Chem. Eng. J. 2022, 428, 131289. [Google Scholar] [CrossRef]
- Silva, R.R.; Raymundo-Pereira, P.A.; Campos, A.M.; Wilson, D.; Otoni, C.G.; Barud, H.S.; Costa, C.A.R.; Domeneguetti, R.R.; Balogh, D.T.; Ribeiro, S.J.L.; et al. Microbial Nanocellulose Adherent to Human Skin Used in Electrochemical Sensors to Detect Metal Ions and Biomarkers in Sweat. Talanta 2020, 218, 121153. [Google Scholar] [CrossRef]
- Shalauddin, M.; Akhter, S.; Basirun, W.J.; Bagheri, S.; Anuar, N.S.; Johan, M.R. Hybrid Nanocellulose/f-MWCNTs Nanocomposite for the Electrochemical Sensing of Diclofenac Sodium in Pharmaceutical Drugs and Biological Fluids. Electrochim. Acta 2019, 304, 323–333. [Google Scholar] [CrossRef]
- Jung, M.; Kim, K.; Kim, B.; Lee, K.J.; Kang, J.W.; Jeon, S. Vertically Stacked Nanocellulose Tactile Sensor. Nanoscale 2017, 9, 17212–17219. [Google Scholar] [CrossRef]
- Ortolani, T.S.; Pereira, T.S.; Assumpção, M.H.M.T.; Vicentini, F.C.; Gabriel de Oliveira, G.; Janegitz, B.C. Electrochemical Sensing of Purines Guanine and Adenine Using Single-Walled Carbon Nanohorns and Nanocellulose. Electrochim. Acta 2019, 298, 893–900. [Google Scholar] [CrossRef]
- Papavasileiou, A.V.; Trachioti, M.G.; Hrbac, J.; Prodromidis, M.I. Simultaneous determination of guanine and adenine in human saliva with graphite sparked screen-printed electrodes. Talanta 2022, 239, 123119. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Cen, Z.; Wei, L. Characterization of Nanocellulose and Activated Carbon Nanocomposite Films’ Biosensing Properties for Smart Packaging. Carbohydr. Polym. 2019, 225, 115189. [Google Scholar] [CrossRef]
- Razalli, R.L.; Abdi, M.M.; Tahir, P.M.; Moradbak, A.; Sulaiman, Y.; Heng, L.Y. Polyaniline-Modified Nanocellulose Prepared from Semantan Bamboo by Chemical Polymerization: Preparation and Characterization. RSC Adv. 2017, 7, 25191–25198. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Sain, M. Flexible Electrically Conductive Films Based on Nanofibrillated Cellulose and Polythiophene Prepared via Oxidative Polymerization. Carbohydr. Polym. 2019, 220, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xing, L.; Hu, C.; Zhang, W.; Lin, X.; Gu, J. Facile synthesis of nanocellulose-based Cu2O/Ag heterostructure as a surface-enhanced Raman scattering substrate for trace dye detection. Int. J. Biol. Macromol. 2022, 205, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xi, J.; Chen, H.; Li, S.; Zhang, L.; Li, P.; Wu, W. Flexible 2D Nanocellulose-Based SERS Substrate for Pesticide Residue Detection. Carbohydr. Polym. 2022, 277, 118890. [Google Scholar] [CrossRef] [PubMed]
- Song, S.W.; Kim, D.; Kim, J.; You, J.; Kim, H.M. Flexible Nanocellulose-Based SERS Substrates for Fast Analysis of Hazardous Materials by Spiral Scanning. J. Hazard. Mater. 2021, 414, 125160. [Google Scholar] [CrossRef]
- Parnsubsakul, A.; Ngoensawat, U.; Wutikhun, T.; Sukmanee, T.; Sapcharoenkun, C.; Pienpinijtham, P.; Ekgasit, S. Silver Nanoparticle/Bacterial Nanocellulose Paper Composites for Paste-and-Read SERS Detection of Pesticides on Fruit Surfaces. Carbohydr. Polym. 2020, 235, 115956. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.M.; Razalli, R.L.; Tahir, P.M.; Chaibakhsh, N.; Hassani, M.; Mir, M. Optimized Fabrication of Newly Cholesterol Biosensor Based on Nanocellulose. Int. J. Biol. Macromol. 2019, 126, 1213–1222. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, G.; Dufresne, A.; Lin, N. Fluorescent Aerogels Based on Chemical Crosslinking between Nanocellulose and Carbon Dots for Optical Sensor. ACS Appl. Mater. Interfaces 2019, 11, 16048–16058. [Google Scholar] [CrossRef] [PubMed]
- Uddin, K.M.A.; Jokinen, V.; Jahangiri, F.; Franssila, S.; Rojas, O.J.; Tuukkanen, S. Disposable Microfluidic Sensor Based on Nanocellulose for Glucose Detection. Glob. Chall. 2019, 3, 1800079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly Stretchable Piezoresistive Graphene-Nanocellulose Nanopaper for Strain Sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef]
- Pang, Z.; Yang, Z.; Chen, Y.; Zhang, J.; Wang, Q.; Huang, F.; Wei, Q. A Room Temperature Ammonia Gas Sensor Based on Cellulose/TiO2/PANI Composite Nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 248–255. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Han, Y.; Zhou, Z.; Yuan, G.; Zhang, X. Cellulose Nanowhisker Modulated 3D Hierarchical Conductive Structure of Carbon Black/Natural Rubber Nanocomposites for Liquid and Strain Sensing Application. Compos. Sci. Technol. 2016, 124, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Zhang, X.; Wu, X.; Wang, S.; Lu, C. Cellulose Nanocrystals Mediated Assembly of Graphene in Rubber Composites for Chemical Sensing Applications. Carbohydr. Polym. 2016, 140, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Palanisamy, S.; Velusamy, V.; Balu, S.; Velmurugan, S.; Yang, T.C.K.; Chen, S.W. Sonochemical Synthesis and Anchoring of Zinc Oxide on Hemin-Mediated Multiwalled Carbon Nanotubes-Cellulose Nanocomposite for Ultra-Sensitive Biosensing of H2O2. Ultrason. Sonochem. 2020, 63, 104917. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Mahnashi, M.H.; Alkahtani, S.A.; El-Wekil, M.M. Nitrogen and Sulfur Co-Doped Graphene Quantum Dots/Nanocellulose Nanohybrid for Electrochemical Sensing of Anti-Schizophrenic Drug Olanzapine in Pharmaceuticals and Human Biological Fluids. Int. J. Biol. Macromol. 2020, 165, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Tanguy, N.R.; Kazemi, K.K.; Hong, J.; Cheung, K.C.; Mohammadi, S.; Gnanasekar, P.; Nair, S.S.; Zarifi, M.H.; Yan, N. Flexible, Robust, and High-Performance Gas Sensors Based on Lignocellulosic Nanofibrils Nicolas. Carbohydr. Polym. 2021, 278, 150562. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Naficy, S.; McConchie, R.; Dehghani, F.; Chandrawati, R. Polydiacetylene-Based Sensors to Detect Food Spoilage at Low Temperatures. J. Mater. Chem. C Mater. 2019, 7, 1919–1926. [Google Scholar] [CrossRef]
- Fu, J.; Li, D.; Li, G.; Huang, F.; Wei, Q. Carboxymethyl Cellulose Assisted Immobilization of Silver Nanoparticles onto Cellulose Nanofibers for the Detection of Catechol. J. Electroanal. Chem. 2015, 738, 92–99. [Google Scholar] [CrossRef]
- Fu, J.; Pang, Z.; Yang, J.; Yang, Z.; Cao, J.; Xu, Y.; Huang, F.; Wei, Q. Hydrothermal Growth of Ag-Doped ZnO Nanoparticles on Electrospun Cellulose Nanofibrous Mats for Catechol Detection. Electroanalysis 2015, 27, 1490–1497. [Google Scholar] [CrossRef]
- Esmaeili, C.; Abdi, M.M.; Mathew, A.P.; Jonoobi, M.; Oksman, K.; Rezayi, M. Synergy Effect of Nanocrystalline Cellulose for the Biosensing Detection of Glucose. Sensors 2015, 15, 24681–24697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Liang, S.; Fan, J.; Sheng, G.; Luo, X. Amperometric Sensing of Nitrite Using a Glassy Carbon Electrode Modified with a Multilayer Consisting of Carboxylated Nanocrystalline Cellulose and Poly (Diallyldimethyl Ammonium) Ions in a PEDOT Host. Microchim. Acta 2016, 183, 2031–2037. [Google Scholar] [CrossRef]

















| Properties | Description | References |
|---|---|---|
| Surface modification |
| [67,68] |
| Nanocellulose structuring in solid films |
| [69,70] |
| Large specific surface area/porous structure |
| [71,72] |
| Tensile |
| [73] |
| Optical/fluorescent |
| [74,75] |
| Electrical |
| [74] |
| Biodegradable |
| [76] |
| Sensing Principles | Magnitude or Signal | Example of Sensing Material | Advantages | References |
|---|---|---|---|---|
| Chemiresistive | Resistance of sensing layer |
|
| [84] |
| Capacitive | Change of dielectric constant of sensing layer at high frequency |
|
| [85] |
| Impedance metric | Change of impedance of sensing layer at high frequency |
|
| [86] |
| Field effect transistor | Change of source-drain current |
|
| [87,88] |
| Mechanical | Change of wave propagates on the surface of the sensing layer |
|
| [89] |
| Optical | Change in the spectrum of the sensing material |
|
| [90,91] |
| Sensing Response Behaviour | Target Analyte | n-Type Sensor | p-Type Sensor |
|---|---|---|---|
| Oxidising analytes | Cl2, N2O, O2, compressed air, H2O2, F2 | Resistance increases | Resistance decreases |
| Reducing analytes | H2, CO, H2S, CH4, NH3, C6H6 | Resistance decreases | Resistance increases |
| Minority carrier | - | Hole (+) | Electron (−) |
| Majority carrier | - | Electron (−) | Hole (+) |
| Composite | Target Analyte | Performances | Advantages | References |
|---|---|---|---|---|
| Silver nanoparticles/BNC | Methomyl |
|
| [111] |
| Polyaniline/CNC (screen-printed electrodes with thin layer ionic liquid) | Cholesterol oxidase |
|
| [112] |
| CNF/carbon dots | Glutaraldehyde |
|
| [113] |
| Nanocellulose/polyethylene terephthalate (PET) | Glucose oxidase |
|
| [114] |
| Graphene-nanocellulose (impregnated in polydimethylsiloxane for stretchable nanopaper) | Bending and stretching |
|
| [115] |
| Material | Target Analyte | Performances | Advantages | References |
|---|---|---|---|---|
| Polydiacetylene/CNC/chitosan | Ammonia |
|
| [122] |
| AgNPs/carboxymethyl cellulose/CNF | Catechol |
|
| [123] |
| Lac/Ag/ZnO/CNF | Catechol |
|
| [124] |
| Peptide/poly(vinyl alcohol)/CNC | Trypsin |
|
| [70] |
| Graphene oxide/PPy/CNC | Glucose |
|
| [125] |
| Poly(diallyldimethyl ammonium chloride/carboxylated CNC/poly(3,4-ethylenedioxythiophene) | Nitrite |
|
| [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norrrahim, M.N.F.; Knight, V.F.; Nurazzi, N.M.; Jenol, M.A.; Misenan, M.S.M.; Janudin, N.; Kasim, N.A.M.; Shukor, M.F.A.; Ilyas, R.A.; Asyraf, M.R.M.; et al. The Frontiers of Functionalized Nanocellulose-Based Composites and Their Application as Chemical Sensors. Polymers 2022, 14, 4461. https://doi.org/10.3390/polym14204461
Norrrahim MNF, Knight VF, Nurazzi NM, Jenol MA, Misenan MSM, Janudin N, Kasim NAM, Shukor MFA, Ilyas RA, Asyraf MRM, et al. The Frontiers of Functionalized Nanocellulose-Based Composites and Their Application as Chemical Sensors. Polymers. 2022; 14(20):4461. https://doi.org/10.3390/polym14204461
Chicago/Turabian StyleNorrrahim, Mohd Nor Faiz, Victor Feizal Knight, Norizan Mohd Nurazzi, Mohd Azwan Jenol, Muhammad Syukri Mohamad Misenan, Nurjahirah Janudin, Noor Azilah Mohd Kasim, Muhammad Faizan A. Shukor, Rushdan Ahmad Ilyas, Muhammad Rizal Muhammad Asyraf, and et al. 2022. "The Frontiers of Functionalized Nanocellulose-Based Composites and Their Application as Chemical Sensors" Polymers 14, no. 20: 4461. https://doi.org/10.3390/polym14204461