Electrospun Porous Nanofibers: Pore−Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater
Abstract
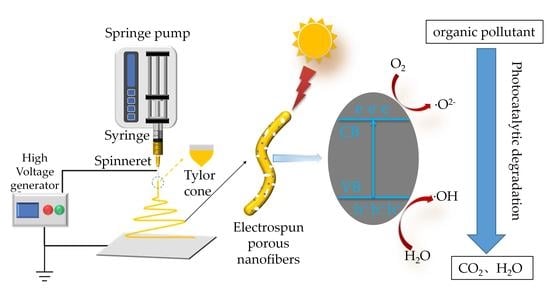
:1. Introduction
2. Pore−Forming Mechanism of Electrospun Porous Nanofibers
2.1. Breath Figures (BFs)
2.2. Vapor Induced Phase Separation (VIPS)
2.3. Non−Solvent Induced Phase Separation (NIPS)
2.4. Thermally Induced Phase Separation (TIPS)
2.5. Selective Removal (SR)
3. Applications in Photocatalytic Degradation of Water Pollutants
3.1. Organic Pollutants
3.1.1. Organic Dyes
3.1.2. Phenolic Pollutants
3.1.3. Antibiotic
3.2. Inorganic Pollutants
3.3. Bacteria
4. Relationships between Porous Structure and the Properties of Nanofibers
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun medical sutures for wound healing: A review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef] [PubMed]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Fibrous polymer-based composites obtained by electrospinning for bone tissue engineering. Polymers 2022, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kaneti, Y.V.; Bando, Y.; Lin, J.; Liu, C.; Li, J.; Yamauchi, Y. Metal–organic framework-derived one-dimensional porous or hollow carbon-based nanofibers for energy storage and conversion. Mater. Horiz. 2018, 5, 394–407. [Google Scholar] [CrossRef]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured electrospun nanofibers for air filtration: A review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, B.; Zhu, L.; Jin, X. Controlling the surface structure, mechanical properties, crystallinity, and piezoelectric properties of electrospun PVDF nanofibers by maneuvering molecular weight. Soft Mater. 2019, 17, 181–189. [Google Scholar] [CrossRef]
- Zaarour, B.; Tina, H.; Zhu, L.; Jin, X. Branched nanofibers with tiny diameters for air filtration via one-step electrospinning. J. Ind. Text. 2020, 152808372092377. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.; Lu, C.; Chai, Y.; Cao, Z.; Lu, J.; Zhang, Z.; Zhao, H.; Huang, Y.Y.; Yao, S.; et al. Aligned fibrin/functionalized self-assembling peptide interpenetrating nanofiber hydrogel presenting multi-cues promotes peripheral nerve functional recovery. Bioact. Mater. 2022, 8, 529–544. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L. Preparation and characterization of porous core-shell fibers for slow release of tea polyphenols. Polymers 2018, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Harding, D.R.; Yang, H. Helical peanut-shaped poly(vinyl pyrrolidone) ribbons generated by electrospinning. Polymer 2013, 54, 6752–6759. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, P.; Yang, Y.Y.; Yu, D.G. Electrospun beads-on-the-string nanoproducts: Preparation and drug delivery application. Curr. Drug Deliv. 2022, 19. [Google Scholar] [CrossRef]
- Iacob, A.T.; Dragan, M.; Ionescu, O.M.; Profire, L.; Ficai, A.; Andronescu, E.; Georgeta, L.; Lupascu, D. An Overview of Biopolymeric Electrospun Nanofibers Based on Polysaccharides for Wound Healing Management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, M.C.; Wu, S.H. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Angel, N.; Li, S.N.; Yan, F.; Kong, L.Y. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhao, P. The Key Elements for Biomolecules to Biomaterials and to Bioapplications. Biomolecules 2022, 12, 1234. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Song, W.; Zhang, Y.; Yu, D.-G.; Liu, Y. Electrospun core (HPMC–acetaminophen)–shell (PVP–sucralose) nanohybrids for rapid drug delivery. Gels 2022, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Lo, T.S.; Lin, Y.T.; Chien, Y.H.; Lu, C.J.; Liu, S.J. Fabrication of drug-eluting polycaprolactone/poly(lactic-co-glycolic acid) prolapse mats using solution-extrusion 3D printing and coaxial electrospinning techniques. Polymers 2021, 13, 2295. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, W.; Feng, Z.; Liu, Y.; Liu, P.; Xie, Y.; Yu, D.G. Electrospun nanofibers for periodontal treatment: A recent progress. Int. J. Nanomed. 2022, 17, 4137–4162. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H. Conductive bicomponent fibers containing polyaniline produced via side-by-side electrospinning. Polymers 2019, 11, 954. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Lu, Z.H.; Zhao, P.; Kang, S.X.; Yang, Y.Y.; Yu, D.G. Modified tri–axial electrospun functional core–shell nanofibrous membranes for natural photodegradation of antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Gao, Y.; Liu, Y.; Yu, D.; Liu, P. Electrospun Core–Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance. Biomolecules 2022, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Lu, X.; Murugadoss, V.; Huang, M.; Yang, H.; Wan, F.; Yu, D.G.; Guo, Z. Electrospun structural nanohybrids combining three composites for fast helicide delivery. Adv. Compos. Hybrid. Mater. 2022. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Yan, C.; Liu, H.; Yu, D.-G. Advances in the Application of Electrospun Drug-Loaded Nanofifibers in the Treatment of Oral Ulcers. Biomolecules 2022, 12, 1254. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, W.; Kaiser, A. Electrospinning of metal–organic frameworks for energy and environmental applications. Adv. Sci. 2020, 7, 1902590. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, S.J.; Hu, Y.M.; Lv, F.Z.; Zhang, Y.H. Preparation of Bi-based porous and magnetic electrospun fibers and their photocatalytic properties in weak polar medium. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 610, 125718. [Google Scholar] [CrossRef]
- Park, K.R.; Cho, H.B.; Lee, J.; Song, Y.; Kim, W.B.; Choa, Y.H. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuators B: Chem. 2020, 302, 127179. [Google Scholar] [CrossRef]
- Wang, B.; Lu, G.; Luo, Q.P.; Wang, T. Free-standing porous carbon nanofiber networks from electrospinning polyimide for supercapacitors. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Miranda, C.S.; Silva, A.F.G.; Pereira-Lima, S.M.M.A.; Costa, S.P.G.; Homem, N.C.; Felgueiras, H.P. Tunable spun fiber constructs in biomedicine: Influence of processing parameters in the fibers’ architecture. Pharmaceutics 2022, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, H.; Zhou, J.; Lin, Z.; Wu, D.; Liu, C.; Cao, Z. Laser induced porous electrospun fibers for enhanced filtration of xylene gas. J. Hazard. Mater. 2020, 399, 122976. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. A conversation with Akira Fujishima. ACS Energy Lett. 2017, 2, 1586–1587. [Google Scholar] [CrossRef]
- Xue, J.; Fujitsuka, M.; Majima, T. The role of nitrogen defects in graphitic carbon nitride for visible-light-driven hydrogen evolution. Phys. Chem. Chem. Phys. 2019, 21, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Akshhayya, C.; Okla, M.K.; AL-ghamdi, A.A.; Abdel-Maksoud, M.A.; AbdElgawad, H.; Das, A.; Khan, S.S. Construction of S-scheme heterojunction CuFe2O4/α-MnO2 with tuned bandgap for enhanced white light harvesting: Insights of photoluminescence, raman scattering and photocatalysis. Surf. Interfaces 2021, 27, 101523. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Leung, W.W.F. Electrospun nanofibers of p-type CuO/n-type TZB-Gr heterojunctions with enhanced photocatalytic activity. Mater. Chem. Phys. 2019, 232, 475–484. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Liu, Y.; Gao, Y.; Kim, H.Y.; Ouyang, Y.; Yu, D.G. Progresses on electrospun metal–organic frameworks nanofibers and their wastewater treatment applications. Mater. Today Chem. 2022, 25, 100974. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, M.; Wang, P.; Xu, X.; Liu, Y.; Yu, D.G. Ingenious construction of Ni(DMG)2/TiO2-decorated porous nanofibers for the highly efficient photodegradation of pollutants in water. Colloids Surf. A: Physicochem. Eng. Aspects. 2022, 650, 129561. [Google Scholar] [CrossRef]
- Leong, M.F.; Chian, K.S.; Mhaisalkar, P.S.; Ong, W.F.; Ratner, B.D. Effect of electrospun poly(d,l-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. J. Biomed. Mater. Res. 2009, 89A, 1040–1048. [Google Scholar] [CrossRef]
- Filova, E.; Blanquer, A.; Knitlova, J.; Plencner, M.; Jencova, V.; Koprivova, B.; Lisnenko, M.; Kostakova, E.K.; Prochazkova, R.; Bacakova, L. The effect of the controlled release of platelet lysate from PVA nanomats on keratinocytes, endothelial cells and fibroblasts. Nanomaterials 2021, 11, 995. [Google Scholar] [CrossRef]
- Tasci, M.E.; Dede, B.; Tabak, E.; Gur, A.; Sulutas, R.B.; Cesur, S.; Ilhan, E.; Lin, C.C.; Paik, P.; Ficai, D.; et al. Production, optimization and characterization of polylactic acid microparticles using electrospray with porous structure. Appl. Sci. 2021, 11, 5090. [Google Scholar] [CrossRef]
- Otsuka, I.; Barrett, C.J. Electrospinning of photo-responsive azo-cellulose: Towards smart fibrous materials. Cellulose 2019, 26, 6903–6915. [Google Scholar] [CrossRef]
- Stodolak-Zych, E.; Dzierzkowska, E.; Matwally, S.; Mikołajczyk, M.; Gajek, M.; Rapacz-Kmita, A. Multifunctional porous membranes with antibacterial properties. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 19–26. [Google Scholar] [CrossRef]
- Ramos, C.; Lanno, G.M.; Laidmäe, I.; Meos, A.; Härmas, R.; Kogermann, K. High humidity electrospinning of porous fibers for tuning the release of drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 880–892. [Google Scholar] [CrossRef]
- Ma, M.; Gupta, M.; Li, Z.; Zhai, L.; Gleason, K.K.; Cohen, R.E.; Rubner, M.F.; Rutledge, G.C. Decorated electrospun fibers exhibiting superhydrophobicity. Adv. Mater. 2007, 19, 255–259. [Google Scholar] [CrossRef]
- Şimşek, M. Tuning surface texture of electrospun polycaprolactone fibers: Effects of solvent systems and relative humidity. J. Mater. Res. 2020, 35, 332–342. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, H.; Zhao, Z.; Han, C.C. Construction of hierarchical structures by electrospinning or electrospraying. Polymer 2012, 53, 546–554. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Ye, C.; Li, Y.; You, J. Gravity-driven ultrafast separation of water-in-oil emulsion by hierarchically porous electrospun poly(l-lactide) fabrics. J. Membr. Sci. 2018, 563, 762–767. [Google Scholar] [CrossRef]
- Gao, J.F.; Hu, M.J.; Li, W.; Wong, J.S.P.; Li, R.K.Y. Morphological evolution from porous nanofibers to rice like nanobeans. Mater. Lett. 2014, 128, 110–113. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Jin, X.; Cheng, J. Polyethylene oxide-coated electrospun polyimide fibrous seperator for high-performance lithium-ion battery. J. Mater. Sci. Technol. 2016, 32, 200–206. [Google Scholar] [CrossRef]
- Mei, L.; Wang, X.; Liu, Y.; Wang, J. Computer simulation of PAN/PVP blends compatibility and preparation of aligned PAN porous nanofibers via magnetic-field-assisted electrospinning PAN/PVP blends. Mater. Sci.-Medzg. 2019, 25, 54–59. [Google Scholar] [CrossRef]
- Huang, C.; Wei, T.; Peng, S.; Lee, K. Study of electrospun polyacrylonitrile fibers with porous and ultrafine nanofibril structures: Effect of stabilization treatment on the resulting carbonized structure. J. Appl. Polym. Sci. 2019, 136, 48218. [Google Scholar] [CrossRef]
- Dong, S.; Yan, S.; Gao, W.; Liu, G.; Hao, H. A phase separation route to synthesize α-Fe2O3 porous nanofibers via electrospinning for ultrafast ethanol Sensing. J. Electron. Mater. 2018, 47, 3934–3941. [Google Scholar] [CrossRef]
- Cho, J.S.; Park, J.S.; Kang, Y.C. Porous FeS nanofibers with numerous nanovoids obtained by kirkendall diffusion effect for use as anode materials for sodium-ion batteries. Nano Res. 2017, 10, 897–907. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, B.; Gong, H.; Li, J. Fabrication of hierarchical porous poly (l-lactide) (PLLA) fibrous membrane by electrospinning. Polymer 2021, 226, 123797. [Google Scholar] [CrossRef]
- Fashandi, H.; Karimi, M. Pore formation in polystyrene fiber by superimposing temperature and relative humidity of electrospinning atmosphere. Polymer 2012, 53, 5832–5849. [Google Scholar] [CrossRef]
- Kong, Q.; Li, Z.G.; Ren, X.H.; Gu, H.; Ma, W.J. The surface morphology and dynamic impact properties with rebounding and splashing of water droplet on phase separation and breath figure assisted electrospinning films. Des. Monomers Polym. 2021, 24, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Zhou, L.; Sun, X.; Du, H.; Bian, X.; Zhu, Z.; Wen, Y. Enzyme-responsive food packaging system based on pectin-coated poly (lactic acid) nanofiber films for controlled release of thymol. Food Res. Int. 2022, 157, 111256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Z.; Liu, L.; Bai, L.; Bao, R.Y.; Yang, M.B.; Yang, W. Degradable ultrathin high-performance photocatalytic hydrogen generator from porous electrospun composite fiber membrane with enhanced light absorption ability. J. Mater. Chem. A 2021, 9, 10277–10288. [Google Scholar] [CrossRef]
- Zaarour, B.; Zhang, W.; Zhu, L.; Jin, X.Y.; Huang, C. Maneuvering surface structures of polyvinylidene fluoride nanofibers by controlling solvent systems and polymer concentration. Text. Res. J. 2019, 89, 2406–2422. [Google Scholar] [CrossRef]
- Kossyvaki, D.; Barbetta, A.; Contardi, M.; Bustreo, M.; Dziza, K.; Lauciello, S.; Athanassiou, A.; Fragouli, D. Highly porous curcumin-loaded polymer mats for rapid detection of volatile amines. ACS Appl. Polym. Mater. 2022, 4, 4464–4475. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Si, Y.; Yu, J.; Ding, B. Super-elastic fluorinated polyurethane nanofibrous membranes with simultaneously waterproof and breathable performance. ACS Appl. Polym. Mater. 2022, 4, 5557–5565. [Google Scholar] [CrossRef]
- Samadian, H.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Bit, A.; Alam, M.; Goodarzi, A.; Darya, G.; Salehi, M. A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and Taurine for bone regeneration. Sci. Rep. 2020, 10, 13366. [Google Scholar] [CrossRef]
- He, T.S.; Yu, X.D.; Bai, T.J.; Li, X.Y.; Fu, Y.R.; Cai, K.D. Porous carbon nanofibers derived from PAA-PVP electrospun fibers for supercapacitor. Ionics 2020, 26, 4103–4111. [Google Scholar] [CrossRef]
- Ahmad, M.A.T.; Abdul Rahman, N. Preparation and characterization of highly porous polyacrylonitrile electrospun nanofibers using lignin as soft template via selective chemical dissolution technique. Polymers 2021, 13, 3938. [Google Scholar] [CrossRef] [PubMed]
- Pavasupree, S.; Srikulkit, K.; Rangkupan, R. Preparation and characterization of PLA-PEO bicomponent fibers with porous-smooth surface by co-electrospinning. Adv. Mater. Res. 2014, 849, 115–120. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Z.; Li, M.M.; Li, R.S.; Fang, T. Hierarchically structured PMMA fibers fabricated by electrospinning. Rsc Adv. 2014, 4, 52973–52985. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Huang, C.; Thomas, N.L. Fabricating porous poly(lactic acid) fibres via electrospinning. Eur. Polym. J. 2018, 99, 464–476. [Google Scholar] [CrossRef]
- Yoon, C.K.; Park, B.K.; Lee, W.I. Characteristics of micro-glass bead/PLA porous composite prepared by electrospinning. Adv. Compos. Mater. 2018, 27, 183–193. [Google Scholar] [CrossRef]
- Altay, P.; Uçar, N. Comparative analysis of sound and thermal insulation properties of porous and non-porous polystyrene submicron fiber membranes. J. Text. Inst. 2021, 113, 2177–2184. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Zhao, K. Fabrication of hollow and porous polystyrene fibrous membranes by electrospinning combined with freeze-drying for oil removal from water. J. Appl. Polym. Sci. 2019, 136, 47262. [Google Scholar] [CrossRef]
- Bae, H.S.; Haider, A.; Selim, K.M.K.; Kang, D.Y.; Kim, E.J.; Kang, I.K. Fabrication of highly porous PMMA electrospun fibers and their application in the removal of phenol and iodine. J Polym Res 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Lim, C.T.; Kotaki, M. Study on structural and mechanical properties of porous PLA nanofibers electrospun by channel-based electrospinning system. Polymer 2015, 56, 572–580. [Google Scholar] [CrossRef]
- Jiang, A.-Y.; Pan, Z.J. Surface-porous and hollow poly(lactic acid) (SPH-PLA) and surface-porous and lotus-root-like PLA (SPL-PLA) nanofibres: Preparation, quantitative analysis, and modelling. J. Nanopart. Res. 2020, 22, 239. [Google Scholar] [CrossRef]
- Lu, P.; Xia, Y.N. Maneuvering the Internal Porosity and Surface Morphology of Electrospun Polystyrene Yarns by Controlling the Solvent and Relative Humidity. Langmuir 2013, 29, 7070–7078. [Google Scholar] [CrossRef]
- Tang, N.; Si, Y.; Yu, J.Y.; Ding, B. Leaf vein-inspired microfiltration membrane based on ultrathin nanonetworks. Environ. Sci.-Nano 2020, 7, 2644–2653. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, G.H.; Li, Y.L.; Fan, G.D. Effects of the glycerophosphate-polylactic copolymer formation on electrospun fibers. Appl. Surf. Sci. 2018, 443, 236–243. [Google Scholar] [CrossRef]
- El-Samak, A.A.; Ponnamma, D.; Hassan, M.K.; Al-Maadeed, M.A. A stable porous vessel for photocatalytic degradation of Azocarmine G dye. Microporous Mesoporous Mater. 2022, 341, 111994. [Google Scholar] [CrossRef]
- Dong, T.; Ul Arifeen, W.; Choi, J.; Yoo, K.; Ko, T. Surface-modified electrospun polyacrylonitrile nano-membrane for a lithium-ion battery separator based on phase separation mechanism. Chem. Eng. J. 2020, 398, 125646. [Google Scholar] [CrossRef]
- Qi, Z.; Yu, H.; Chen, Y.; Zhu, M. Highly porous fibers prepared by electrospinning a ternary system of nonsolvent/solvent/poly(l-lactic acid). Mater. Lett. 2009, 63, 415–418. [Google Scholar] [CrossRef]
- Prasad, G.; Liang, J.W.; Zhao, W.; Yao, Y.; Tao, T.; Liang, B.; Lu, S.G. Enhancement of solvent uptake in porous PVDF nanofibers derived by a water-mediated electrospinning technique. J. Mater. 2021, 7, 244–253. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Yuan, X.; Wang, T. Porous polyacrylonitrile/graphene oxide nanofibers designed for high efficient adsorption of chromium ions (VI) in aqueous solution. Chem. Eng. J. 2020, 392, 123730. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Lee, J.K.Y.; Ji, D.; Srinivasan, M.; Ramakrishna, S. Electrospun hollow nanofibers for advanced secondary batteries. Nano Energy 2017, 39, 111–139. [Google Scholar] [CrossRef]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef]
- Yu, X.; Xiang, H.; Long, Y.; Zhao, N.; Zhang, X.; Xu, J. Preparation of porous polyacrylonitrile fibers by electrospinning a ternary system of PAN/DMF/H2O. Mater. Lett. 2010, 64, 2407–2409. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.G.; Chanunpanich, N.; Park, J.S. Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int. J. Pharm. 2012, 439, 296–306. [Google Scholar] [CrossRef]
- Song, J.; Zhang, B.; Lu, Z.; Xin, Z.; Liu, T.; Wei, W.; Zia, Q.; Pan, K.; Gong, R.H.; Bian, L.; et al. Hierarchical porous poly(l-lactic acid) nanofibrous membrane for ultrafine particulate aerosol filtration. ACS Appl. Mater. Interfaces 2019, 11, 46261–46268. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Zhou, K.; Zhang, X.; Ullah, I.; Ou, H.; Zhang, W.; Wu, T. Facile fabrication of porous polymer fibers via cryogenic electrospinning system. J. Mater. Process. Technol. 2019, 266, 551–557. [Google Scholar] [CrossRef]
- Ye, X.Y.; Lin, F.W.; Huang, X.J.; Liang, H.Q.; Xu, Z.K. Polymer fibers with hierarchically porous structure: Combination of high temperature electrospinning and thermally induced phase separation. Rsc Adv. 2013, 3, 13851–13858. [Google Scholar] [CrossRef]
- McCann, J.T.; Marquez, M.; Xia, Y.N. Highly porous fibers by electrospinning into a cryogenic liquid. J. Am. Chem. Soc. 2006, 128, 1436–1437. [Google Scholar] [CrossRef] [PubMed]
- Bayley, G.M.; Mallon, P.E. Porous microfibers by the electrospinning of amphiphilic graft copolymer solutions with multi-walled carbon nanotubes. Polymer 2012, 53, 5523–5539. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Co-continuous nanoscale assembly of Nafion-polyacrylonitrile blends within nanofibers: A facile route to fabrication of porous nanofibers. Soft Matter 2013, 9, 846–852. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Guan, J.M.; Wang, X.F.; Yu, J.Y.; Ding, B. Balsam-Pear-Skin-Like Porous Polyacrylonitrile Nanofibrous Membranes Grafted with Polyethyleneimine for Postcombustion CO2 Capture. Acs Appl. Mater. Interfaces 2017, 9, 41087–41098. [Google Scholar] [CrossRef]
- Yan, S.; Luo, S.; Wang, Q.; Zhang, Y.; Liu, X. Rational design of hierarchically sulfide and MXene-reinforced porous carbon nanofibers as advanced electrode for high energy density flexible supercapacitors. Compos. Part B: Eng. 2021, 224, 109246. [Google Scholar] [CrossRef]
- Zhang, C.; Nicolosi, V. Graphene and MXene-based transparent conductive electrodes and supercapacitors. Energy Storage Mater. 2019, 16, 102–125. [Google Scholar] [CrossRef]
- Xia, Y.; Mathis, T.S.; Zhao, M.Q.; Anasori, B.; Dang, A.; Zhou, Z.; Cho, H.; Gogotsi, Y.; Yang, S. Thickness-independent capacitance of vertically aligned liquid-crystalline MXenes. Nature 2018, 557, 409–412. [Google Scholar] [CrossRef]
- Methaapanon, R.; Chutchakul, K.; Pavarajarn, V. Photocatalytic zinc oxide on flexible polyacrylonitrile nanofibers via sol–gel coaxial electrospinning. Ceram. Int. 2020, 46, 8287–8292. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, X.; Yang, H.; Xu, Z.L.; Wei, Y.M. Preparation of porous PVDF nanofiber coated with Ag NPs for photocatalysis application. Fibers Polym 2016, 17, 21–29. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, F.; Wang, Y.; Yin, L.; Li, J.; Huang, J.; Kong, X.; Feng, Q. Building {0001} and {101¯1} facet heterojunctions on hexagonal pyramid CdS single crystals with high photoactivity and photostability for hydrogen evolution. Chem. Eng. J. 2021, 426, 130777. [Google Scholar] [CrossRef]
- Munyai, S.; Tetana, Z.N.; Mathipa, M.M.; Ntsendwana, B.; Hintsho-Mbita, N.C. Green synthesis of cadmium sulphide nanoparticles for the photodegradation of malachite green dye, sulfisoxazole and removal of bacteria. Optik 2021, 247, 167851. [Google Scholar] [CrossRef]
- Liu, Q.; Zhan, H.; Huang, X.; Song, Y.; He, S.; Li, X.; Wang, C.; Xie, Z. High visible light photocatalytic activity of SnO2-x nanocrystals with rich oxygen vacancy. Eur. J. Inorg. Chem. 2021, 2021, 4370–4376. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y.; Wang, Y.; Li, H.; Zhang, R. Facile hydrothermal synthesis of SnO2 quantum dots with enhanced photocatalytic degradation activity: Role of surface modification with chloroacetic acid. J. Environ. Chem. Eng. 2021, 9, 105618. [Google Scholar] [CrossRef]
- Huang, J.; Liu, S.; Long, W.; Wang, Q.; Yu, X.; Li, S. Highly enhanced photodegradation of emerging pollutants by Ag/AgCl/Ta2O5−x mesocrystals. Sep. Purif. Technol. 2021, 279, 119733. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Q.; Xiao, C.; You, Y.; Zhang, C.; Liu, H. Supported electrospun ultrafine fibrous poly (tetrafluoroethylene)/ZnO porous membranes and their photocatalytic applications. Chem. Eng. Technol. 2018, 41, 656–662. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Zhu, L.; Hong, M.; Wei Ho, G. Hierarchical assembly of SnO2/ZnO nanostructures for enhanced photocatalytic performance. Sci Rep 2015, 5, 11609. [Google Scholar] [CrossRef]
- Elkady, M.F.; Hassan, H.S. Photocatalytic degradation of malachite green dye from aqueous solution using environmentally compatible Ag/ZnO polymeric nanofibers. Polymers 2021, 13, 2033. [Google Scholar] [CrossRef]
- Zhou, T.T.; Zhao, F.H.; Cui, Y.Q.; Chen, L.X.; Yan, J.S.; Wang, X.X.; Long, Y.Z. Flexible TiO2/PVDF/g-C3N4 nanocomposite with excellent light photocatalytic performance. Polymers 2020, 12, 55. [Google Scholar] [CrossRef]
- Bai, L.; Huang, H.; Yu, S.; Zhang, D.; Huang, H.; Zhang, Y. Role of transition metal oxides in g-C3N4-based heterojunctions for photocatalysis and supercapacitors. J. Energy Chem. 2022, 64, 214–235. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F. Under vacuum synthesis of type-I heterojunction between red phosphorus and graphene like carbon nitride with enhanced catalytic, electrochemical and charge separation ability for photodegradation of an acute toxicity category-III compound. Appl. Catal. B: Environ. 2018, 238, 568–575. [Google Scholar] [CrossRef]
- Lei, X.; Yin, X.; Meng, S.; Li; Wang, H.; Xi, H.; Yang, J.; Xu, X.; Yang, Z.; Lei, Z. Fabrication of type II heterojunction in ZnIn2S4@ZnO photocatalyst for efficient oxidative coupling of benzylamine under visible light. Mol. Catal. 2022, 519, 112120. [Google Scholar] [CrossRef]
- Pratibha; Kapoor, A.; Rajput, J.K. Nanostructured materials for the visible-light driven hydrogen evolution by water splitting: A review. Int. J. Hydrog. Energy 2022, 47, 17544–17582. [Google Scholar] [CrossRef]
- Shen, J.; Qian, L.; Huang, J.; Guo, Y.; Zhang, Z. Enhanced degradation toward levofloxacin under visible light with S-scheme heterojunction In2O3/Ag2CO3: Internal electric field, DFT calculation and degradation mechanism. Sep. Purif. Technol. 2021, 275, 119239. [Google Scholar] [CrossRef]
- Du, M.; Cao, S.; Ye, X.; Ye, J. Recent advances in the fabrication of all-solid-state nanostructured TiO2-based Z-scheme heterojunctions for environmental remediation. J. Nanosci. Nanotechnol. 2020, 20, 5861–5873. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, F.; Nezamzadeh-Ejhieh, A. Study of the photocatalytic activity of CdS–ZnS nano-composite in the photodegradation of rifampin in aqueous solution. J. Mater. Res. Technol. 2020, 9, 16237–16251. [Google Scholar] [CrossRef]
- Wu, S.; Yu, X.; Zhang, J.; Zhang, Y.; Zhu, Y.; Zhu, M. Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal. Chem. Eng. J. 2021, 411, 128555. [Google Scholar] [CrossRef]
- Das, A.; Patra, M.; Kumar, P.M.; Bhagavathiachari, M.; Nair, R.G. Role of type II heterojunction in ZnO–In2O3 nanodiscs for enhanced visible-light photocatalysis through the synergy of effective charge carrier separation and charge transport. Mater. Chem. Phys. 2021, 263, 124431. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, W.; Zhu, L.; Zou, W.; Liu, H.; Fu, Z.; Lu, Y. Synthesis of Au@Bi6Fe2Ti3O18 nanofibers and the corona-poling enhanced visible-light-driven photocatalysis. Mater. Lett. 2019, 241, 115–118. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Ali, S.; Sriubas, M.; Varnagiris, S.; Tuckute, S.; Abdelnaby, M.A.; Kamel, B.M. Highly efficient visible light photodegradation of Cr(VI) using electrospun MWCNTs-Fe3O4@PES nanofibers. Catalysts 2021, 11, 868. [Google Scholar] [CrossRef]
- Li, Q.-H.; Dong, M.; Li, R.; Cui, Y.-Q.; Xie, G.-X.; Wang, X.-X.; Long, Y.-Z. Enhancement of Cr(VI) removal efficiency via adsorption/photocatalysis synergy using electrospun chitosan/g-C3N4/TiO2 nanofibers. Carbohydr. Polym. 2021, 253, 117200. [Google Scholar] [CrossRef]
- Fang, W.; Yu, L.; Xu, L. Preparation, characterization and photocatalytic performance of heterostructured CuO–ZnO-loaded composite nanofiber membranes. Beilstein J. Nanotechnol. 2020, 11, 631–650. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, K.; Xing, G.; Zheng, W.; Tang, Y. One-step synthesis of unique thorn-like BaTiO3–TiO2 composite nanofibers to enhance piezo-photocatalysis performance. Ceram. Int. 2021, 47, 7278–7284. [Google Scholar] [CrossRef]
- Mohamed, A.; Nasser, W.S.; Kamel, B.M.; Hashem, T. Photodegradation of phenol using composite nanofibers under visible light irradiation. Eur. Polym. J. 2019, 113, 192–196. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Han, P. Electrospinning preparation of g-C3N4/Nb2O5 nanofibers heterojunction for enhanced photocatalytic degradation of organic pollutants in water. Sci. Rep. 2021, 11, 22950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, J.; Dai, Q.; Yan, Y.; Yao, B.; Ng, C.C.; Han, F.; Ma, M.; Cheah, P.; Proctor, G.; et al. Enhanced visible-light photocatalytic activity of one dimensional In2O3/In2TiO5 nanobelts. Mater. Res. Bull. 2019, 113, 102–108. [Google Scholar] [CrossRef]
- Bairamis, F.; Konstantinou, I. WO3 fibers/g-C3N4 Z-scheme heterostructure photocatalysts for simultaneous oxidation/reduction of phenol/Cr (VI) in aquatic media. Catalysts 2021, 11, 792. [Google Scholar] [CrossRef]
- Rongan, H.; Haijuan, L.; Huimin, L.; Difa, X.; Liuyang, Z. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar] [CrossRef]
- Darbandi, M.; Eynollahi, M.; Badri, N.; Mohajer, M.F.; Li, Z.-A. NiO nanoparticles with superior sonophotocatalytic performance in organic pollutant degradation. J. Alloy. Compd. 2021, 889, 161706. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Hameed, P.; Rao, R.P.; Shrivastava, N.; Mittal, J.; Mohapatra, S. Biosynthesis of Highly Stable Fluorescent Selenium Nanoparticles and the Evaluation of Their Photocatalytic Degradation of Dye. Bionanoscience 2020, 10, 389–396. [Google Scholar] [CrossRef]
- Abbas, A.; Ahmad, T.; Hussain, S.; Noman, M.; Shahzad, T.; Iftikhar, A.; Cheema; Ijaz, M.; Tahir, M.; Gohari, G.; et al. Immobilized biogenic zinc oxide nanoparticles as photocatalysts for degradation of methylene blue dye and treatment of textile effluents. Int. J. Environ. Sci. Technol. 2022, 1–14. [Google Scholar] [CrossRef]
- Wu, J.; Yi, S.; Wang, Y.; Yao, J.; Gao, W. Polymer-based TiO2 nanocomposite membrane: Synthesis and organic pollutant removal. Int. J. Smart Nano Mater. 2021, 12, 129–145. [Google Scholar] [CrossRef]
- Stoilova, O.; Manolova, N.; Rashkov, I. Electrospun poly(methyl methacrylate)/TiO2 composites for photocatalytic water treatment. Polymers 2021, 13, 3923. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Ma, L.; Xiang, C.; Li, L. TiO2-doped chitosan microspheres supported on cellulose acetate fibers for adsorption and photocatalytic degradation of methyl orange. Polymers 2019, 11, 1293. [Google Scholar] [CrossRef]
- Cinelli, G.; Cuomo, F.; Ambrosone, L.; Colella, M.; Ceglie, A.; Venditti, F.; Lopez, F. Photocatalytic degradation of a model textile dye using Carbon-doped titanium dioxide and visible light. J. Water Process Eng. 2017, 20, 71–77. [Google Scholar] [CrossRef]
- Kebede, W.L.; Kuo, D.H.; Bekena, F.T.; Duresa, L.W. Highly efficient In-Mo(O,S)(2) oxy-sulfide for degradation of organic pollutants under visible light irradiation: An example of photocatalyst on its dye selectivity. Chemosphere 2020, 254. [Google Scholar] [CrossRef]
- Tu, H.; Li, D.; Yi, Y.; Liu, R.; Wu, Y.; Dong, X.; Shi, X.; Deng, H. Incorporation of rectorite into porous polycaprolactone/TiO2 nanofibrous mats for enhancing photocatalysis properties towards organic dye pollution. Compos. Commun. 2019, 15, 58–63. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhu, J.L.; Wen, W.; Zhang, X.H.; Wang, S.F. Spherical covalent organic framework supported Cu/Ag bimetallic nanoparticles with highly catalytic activity for reduction of 4-nitrophenol. J. Solid State Chem. 2022, 311. [Google Scholar] [CrossRef]
- Peng, X.H.; Wang, Y.; Luo, Z.; Zhang, B.W.; Mei, X.L.; Yang, X.P. Facile synthesis of fluorescent sulfur quantum dots for selective detection of p-nitrophenol in water samples. Microchem. J. 2021, 170. [Google Scholar] [CrossRef]
- Pradhan, A.C.; Uyar, T. Morphological control of mesoporosity and nanoparticles within Co3O4–CuO electrospun nanofibers: Quantum confinement and visible light photocatalysis performance. ACS Appl. Mater. Interfaces 2017, 9, 35757–35774. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Guo, D.Z.; Xu, X.C.; Pan, J.M.; Niu, X.H. Colorimetric quantification and discrimination of phenolic pollutants based peroxidase-like Fe3O4 nanoparticles. Sens. Actuators B-Chem. 2020, 303, 127225. [Google Scholar] [CrossRef]
- Li, S.; Shen, X.; Liu, J.; Zhang, L. Synthesis of Ta3N5/Bi2MoO6 core–shell Fiber-shaped heterojunctions as efficient and easily recyclable photocatalysts. Environ. Sci. Nano 2017, 4, 1155–1167. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Lin, S.; Zhou, X.; Dong, H.; Zhan, Y. Fate of antibiotics during membrane separation followed by physical-chemical treatment processes. Sci. Total Environ. 2021, 759, 143520. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lei, X.Y.; Chen, Q.L.; Kang, C.; Li, W.X.; Liu, B.J. Enhanced photocatalytic degradation of tetracycline hydrochloride by novel porous hollow cube ZnFe2O4. J. Photochem. Photobiol. A-Chem. 2018, 364, 794–800. [Google Scholar] [CrossRef]
- Lu, Q.; Dong, C.C.; Wei, F.; Li, J.D.; Wang, Z.; Mu, W.; Han, X.J. Rational fabrication of Bi2WO6 decorated TiO2 nanotube arrays for photocatalytic degradation of organic pollutants. Mater. Res. Bull. 2022, 145. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Xu, K.; Jiang, W.; Liu, Y.; Leng, Z.; Liu, J. Construction of fiber-shaped silver oxide/tantalum nitride p-n heterojunctions as highly efficient visible-light-driven photocatalysts. J. Colloid Interface Sci. 2017, 504, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Tian, X.; Lin, L.; Ding, R.; Yan, W.; Zhao, F. Functional role of mixed-culture microbe in photocatalysis coupled with biodegradation: Total organic carbon removal of ciprofloxacin. Sci. Total Environ. 2021, 784, 147049. [Google Scholar] [CrossRef]
- Song, H.C.; Sun, J.; Shen, T.T.; Deng, L.; Wang, X.K. Insights into the Mechanism of the Bi/BiVO4 Composites for Improved Photocatalytic Activity. Catalysts 2021, 11, 489. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Al-Jendeel, H.A.; Rashid, S.A.; Al-Batty, S. Antibiotics adsorption from contaminated water by composites of ZSM-5 zeolite nanocrystals coated carbon. J. Water Process Eng. 2022, 47, 102745. [Google Scholar] [CrossRef]
- Gupta, B.; Gupta, A.K.; Tiwary, C.S.; Ghosal, P.S. A multivariate modeling and experimental realization of photocatalytic system of engineered S–C3N4/ZnO hybrid for ciprofloxacin removal: Influencing factors and degradation pathways. Environ. Res. 2021, 196, 110390. [Google Scholar] [CrossRef]
- Rong, F.; Lu, Q.; Mai, H.; Chen, D.; Caruso, R.A. Hierarchically porous WO3/CdWO4 fiber-in-tube nanostructures featuring readily accessible active sites and enhanced photocatalytic effectiveness for antibiotic degradation in water. ACS Appl. Mater. Interfaces 2021, 13, 21138–21148. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, K.; Xu, W.; Chen, D.; Fang, J.; Zhu, X.; Sun, J.; Liang, Y.; Hu, X.; Li, R.; et al. Adsorption-photocatalysis synergistic removal of contaminants under antibiotic and Cr(VI) coexistence environment using non-metal g-C3N4 based nanomaterial obtained by supramolecular self-assembly method. J. Hazard. Mater. 2021, 404, 124171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; An, H.; Lin, J.; Feng, M.; Murugadoss, V.; Ding, T.; Liu, H.; Shao, Q.; Mai, X.; Wang, N.; et al. Progress on the photocatalytic reduction removal of chromium contamination. Chem. Rec. 2019, 19, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Xu, F.; Zhang, P.; Xu, Y.; Zhang, G. Green synthesis of poly(pyrrole methane)-based adsorbent for efficient removal of chromium(VI) from aqueous solution. J. Clean. Prod. 2021, 293, 126197. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Zhang, Y.; Gao, K.; Dai, L.; Shen, M.; Wang, W.; Wang, J.; Ni, Y. Cellulose-based electrospun nanofiber membrane with core-sheath structure and robust photocatalytic activity for simultaneous and efficient oil emulsions separation, dye degradation and Cr(VI) reduction. Carbohydr. Polym. 2021, 258, 117676. [Google Scholar] [CrossRef]
- He, J.; Zheng, Z.; Lo, I.M.C. Different responses of gram-negative and gram-positive bacteria to photocatalytic disinfection using solar-light-driven magnetic TiO2-based material, and disinfection of real sewage. Water Res. 2021, 207, 117816. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.; Kim, I.; Song, D. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022. [Google Scholar] [CrossRef]
- Tzeng, J.H.; Weng, C.H.; Yen, L.T.; Gaybullaev, G.; Chang, C.J.; de Luna, M.D.G.; Lin, Y.T. Inactivation of pathogens by visible light photocatalysis with nitrogen-doped TiO2 and tourmaline-nitrogen co-doped TiO2. Sep. Purif. Technol. 2021, 274, 118979. [Google Scholar] [CrossRef]
- Sato, T.; Taya, M. Copper-aided photo sterilization of microbial cells on TiO2 film under irradiation from a white light fluorescent lamp. Biochem. Eng. J. 2006, 30, 199–204. [Google Scholar] [CrossRef]
- Arellano, U.; Wang, J.A.; Balcázar, L.M.; Chen, L.; Salmones, J.; Solís, S.; Asomoza, M.; González, J. Ag/CeO2/SBA-15 hybrid catalysts for the elimination of E. coli in potable water system. J. Appl. Res. Technol. 2020, 18, 315–332. [Google Scholar] [CrossRef]
- Han, N.; Wang, W.; Lv, X.; Zhang, W.; Yang, C.; Wang, M.; Kou, X.; Li, W.; Dai, Y.; Zhang, X. Highly efficient purification of multicomponent wastewater by electrospinning kidney-bean-skin-like porous H-PPAN/RGO-g-PAO@Ag+/Ag composite nanofibrous membranes. ACS Appl. Mater. Interfaces 2019, 11, 46920–46929. [Google Scholar] [CrossRef]
- Ponnamma, D.; Elgawady, Y.; Nair, S.S.; Hassan, M.K.; Al-Maadeed, M.A.A. Core-shell nanofibers of polyvinyl alcohol/polylactic acid containing TiO2 nanotubes for natural sunlight driven photocatalysis. Macromol. Mater. Eng. 2022, 307, 2100482. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Zhang, M.; Wang, M.; Zhou, Y.; Liu, Y.; Yu, D.G. Recent progresses of electrospun nanofibers and their applications in treating heavy metal wastewater. Front. Chem. Sci. Eng. 2022, 17, 1–34. [Google Scholar]
- You, J.; Sheng, W.; Huang, K.; Hou, C.; Yue, H.; Hu, B.; Wang, M.; Wei, D.; Li, Q.; Zhao, L.; et al. Novel cigarlike TiO2 nanofibers: Fabrication, improved mechanical, and electrochemical performances. ACS Appl. Mater. Interfaces 2013, 5, 2278–2282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, C.; Pan, Z. Porous bead-on-string poly(lactic acid) fibrous membranes for air filtration. J. Colloid Interface Sci. 2015, 441, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, D.G.; Williams, G.R.; Bligh, S.W.A. Co-loading of inorganic nanoparticles and natural oil in the electrospun janus nanofibers for a synergetic antibacterial effect. Pharmaceutics 2022, 14, 1208. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, X.; Fu, J.; Yu, D.-G.; Liu, Y. Recent Progress in Electrospun Polyacrylonitrile Nanofifiber-Based Wound Dressing. Polymers 2022, 14, 3266. [Google Scholar] [CrossRef]
- Ji, Y.; Song, W.; Xu, L.; Yu, D.-G.; Annie-Bligh, S.W. A review on electrospun poly(amino acid) nano-fibers and their applications of hemostasis and wound healing. Biomolecules 2022, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhao, P.; Song, W.; Wang, M.; Yu, D.-G. Electrospun Zein/Polyoxyethylene Core-Sheath Ultrathin Fibers and Their Antibacterial Food Packaging Applications. Biomolecules 2022, 12, 1110. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Liu, P.; Yu, D.G.; Ge, R. Electrospun nanofibers-based glucose sensors for glucose detection. Front. Chem. 2022, 10, 944428. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, X.; Liu, P.; Zhang, Y.; Song, W.; Yu, D.-G.; Lu, X. Electrospun healthcare nanofibers from medicinal liquor of Phellinus igniarius. Adv. Compos. Hybrid Mater. 2022, 5, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Yang, Z.; Chen, X.; Yu, D.G.; Shao, J. Hybrid films prepared from a combination of electrospinning and casting for offering a dual-phase drug release. Polymers 2022, 14, 2132. [Google Scholar] [CrossRef] [PubMed]













| Fiber Membrane Composition | Types of Heterojunctions | Photocatalytic Reactants | Photocatalytic Efficiency | References | ||
|---|---|---|---|---|---|---|
| Fiber Skeleton | Photocatalytic Particles | Conversion Rate | Repeatability | |||
| Bi6Fe2Ti3O18 (BFTO) | Au@ (BFTO) | − | RhB | 90% | ≥4 run | [119] |
| Polyethersulfones (PES) | (MWCNTs)−Fe3O4 | − | Cr(VI) | 99% | ≥5 run | [120] |
| Chitosan | g−C3N4/TiO2 | − | Cr(VI) | 90% | ≥5 run | [121] |
| PVDF/PAN | CuO−ZnO | type I | MO | 90% | ≥5 run | [122] |
| BaTiO3–TiO2 | BaTiO3–TiO2 | type II | RhB | 99.8% | ≥5 run | [123] |
| PAN | CNT/TiO2−NH2 | type II | Phenol | 99.7% | −− | [124] |
| g−C3N4/Nb2O5 | g−C3N4/Nb2O5 | − | RhB, phenol | RhB: 98.1%, Phenol: 100% | ≥4 run | [125] |
| In2TiO | In2O3/In2TiO | type II | RhB, Lvofloxacin (LEV) | RhB: 97.1%, LEV: 86% | ≥5 run | [126] |
| g−C3N4 | WO3/g−C3N4 | Z−scheme | Phenol, Cr(VI) | Phenol: 75%, Cr(VI): 90% | ≥3 run | [127] |
| TiO2 | Bi2O3/TiO2 | S−scheme | Phenol | Phenol: 50% | − | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun Porous Nanofibers: Pore−Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater. Polymers 2022, 14, 3990. https://doi.org/10.3390/polym14193990
Cao X, Chen W, Zhao P, Yang Y, Yu D-G. Electrospun Porous Nanofibers: Pore−Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater. Polymers. 2022; 14(19):3990. https://doi.org/10.3390/polym14193990
Chicago/Turabian StyleCao, Xianyang, Wei Chen, Ping Zhao, Yaoyao Yang, and Deng-Guang Yu. 2022. "Electrospun Porous Nanofibers: Pore−Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater" Polymers 14, no. 19: 3990. https://doi.org/10.3390/polym14193990