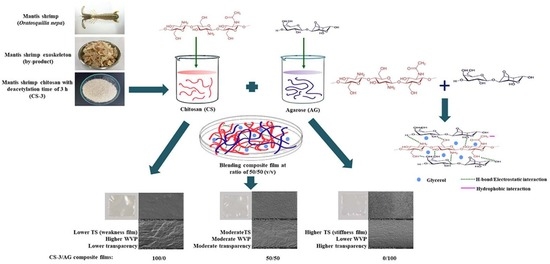
Extraction and Physico–Chemical Characterization of Chitosan from Mantis Shrimp (Oratosquilla nepa) Shell and the Development of Bio-Composite Film with Agarose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals/Bacterial Stains
2.2. Collection and Preparation of Mantis Shrimp Shell
2.3. Preparation of CS
2.4. Characterization of CS
2.4.1. Appearance and Color
2.4.2. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopic Analysis and Determination of Degree of Deacetylation (DDA)
2.4.3. Determination of Intrinsic Viscosity [η] and Viscosity-Average Molecular Weight (Mv)
2.4.4. Determination of Antimicrobial Activity
2.5. Preparation and Characterization of CS/AG Composite Films at Different Ratios
2.5.1. Preparation of CS−3/AG Composite Film
2.5.2. Film Analyses
Film Thickness
TS and EAB
Water Vapor Permeability (WVP)
Color and Transparency
2.5.3. Characterization of Selected Films
ATR-FTIR Spectroscopic Analysis
Morphology Study
2.6. Statistical Analyses
3. Results and discussion
3.1. Extraction Yield
3.2. Characterization of CS as Affected by Deacetylation Times
3.2.1. Appearance and Color
3.2.2. ATR-FTIR and DDA
3.2.3. Intrinsic Viscosity and Viscosity-Average Molecular Weight
3.2.4. Antimicrobial Activity
3.3. Characterization of CS−3/AG Composite Films in Different Ratios
3.3.1. Film Thickness
3.3.2. Mechanical Properties
3.3.3. Water Vapor Permeability (WVP)
3.3.4. Color, Appearance and Transparency
3.4. Characterization of the Selected CS−3/AG Composite Film
3.4.1. FTIR Spectra Analysis
3.4.2. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiransuchalert, R.; Oonwiset, N.; Imarom, Y.; Chindudsadeegul, P.; Laongmanee, P.; Arnupapboon, S. Extraction and characterization of pepsin-soluble collagen from different mantis shrimp species. Fish. Aquat. Sci. 2021, 24, 406–414. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.A. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Antonino, R.S.C.M.; Lia-Fook, B.R.P.; Oliveira-Lima, V.A.; Farias-Rached, R.; Lima, E.P.N.; Silva-Lima, R.J.; Peniche Covas, C.A.; Lia-Fook, M.V. preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Amoo, K.; Olafadehan, O.; Ajayi, T. Optimization studies of chitin and chitosan production from Penaeus notialis shell waste. Afr. J. Biotechnol. 2019, 18, 670–688. [Google Scholar]
- Zamri, A.; Latiff, N.; Abdullah, Q.; Ahmad, F. Extraction and optimization of chitosan from razor clam (Ensis arcuatus) shells by using response surface methodology (RSM). Food Res. 2020, 4, 674–678. [Google Scholar] [CrossRef]
- Yaghobi, N. Controlling chitosan’s molecular weight via multistage deacetylation. Biotechnol. Bioprocess Eng. 2012, 17, 812–817. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Ultrasound-assisted extraction of chitosan from squid pen: Molecular characterization and fat binding capacity. J. Food Sci. 2019, 84, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Wang, K.; Du, L.; Zhang, C.; Lu, Z.; Lu, F.; Zhao, H. Preparation of chitosan/curdlan/carboxymethyl cellulose blended film and its characterization. J. Food Sci. Technol. 2019, 56, 5396–5404. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Hong, P.; Liao, M.; Kong, S.; Huang, N.; Ou, C.; Li, S. Preparation and characterization of chitosan-agarose composite films. Materials 2016, 9, 816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knidri, H.E.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Singh, A.; Aluko, R.E.; Benjakul, S. Pacific white shrimp (Litopenaeus vannamei) shell chitosan and the conjugate with epigallocatechin gallate: Antioxidative and antimicrobial activities. J. Food Biochem. 2021, 45, e13569. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Lianga, S.X.T.; Wong, L.S.; Lim, Y.M.; Djearamanea, S.; Lee, P.F. Effects of Zinc oxide nanoparticles on Streptococcus pyogenes. S. Afr. J. Chem. Eng. 2020, 34, 63–71. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Jaisan, C.; Rawdkuen, S.; Tongdeesoontorn, W.; Klunklin, W. Carboxymethyl cellulose from Young Palmyra palm fruit husk: Synthesis, characterization, and film properties. Food Hydrocoll. 2022, 124, 107277. [Google Scholar] [CrossRef]
- Nuthong, P.; Benjakul, S.; Prodpran, T. Characterization of porcine plasma protein-based films as affected by pretreatment and cross-linking agents. Int. J. Biol. Macromol. 2009, 44, 143–148. [Google Scholar] [CrossRef]
- Jaganathan, K.; Soundarapandian, P.; TV, S.K.; Raffi, S. Extraction and characterization of chitin from marine bycatch crustaceans employing chemical method. IAJMR 2015, 1, 94–102. [Google Scholar]
- Kumari, S.; Rath, P.; Kumar, A.S.H.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Horst, M.N.; Freeman, J.A. The Crustacean Integument: Morphology and Biochemistry, 1st ed.; CRC Press: Boca Raton, FL, USA, 1993; 240p. [Google Scholar]
- Rojsitthisak, P.; How, N.; Chandrkrachang, S.; Stevens, W. Effect of chemical treatment on the characteristics of shrimp chitosan. J. Met. Mater. Miner. 2002, 12, 11–18. [Google Scholar]
- Cui, D.; Yang, J.; Lu, B.; Deng, L.; Shen, H. Extraction and characterization of chitin from Oratosquilla oratoria shell waste and its application in Brassica campestris L.ssp. Int. J. Biol. Macromol. 2022, 198, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.P.; Meireles, M.Â.; Lopes, B.L.; Cabral, F.A. Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Food Eng. 2011, 102, 87–93. [Google Scholar] [CrossRef]
- Okada, S.; Nur, A.; Yamaguchi, K. Carotenoid composition in the exoskeleton of commercial black tiger prawns. Fish. Sci. 1994, 60, 213–215. [Google Scholar] [CrossRef] [Green Version]
- Tokatlı, K.; Demirdöven, A. Optimization of chitin and chitosan production from shrimp wastes and characterization. J. Food Process. Preserv. 2018, 42, e13494. [Google Scholar] [CrossRef]
- Rasweefali, M.K.; Sabu, S.; Sunooj, K.V.; Sasidharan, A.; Xavier, K.A.M. Consequences of chemical deacetylation on physicochemical, structural and functional characteristics of chitosan extracted from deep-sea mud shrimp. Carbohydr. Polym. 2021, 2, 100032. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S. Role of CaCO3 in the physicochemical properties of crustacean-sourced structural polysaccharides. Mater. Chem. Phys. 2016, 184, 203–209. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, X.; Miao, J.; Leng, K. Chitin from antarctic krill shell: Eco-preparation, detection, and characterization. Int. J. Biol. Macromol. 2020, 164, 4125–4137. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S., Jr.; Dutta, P.K.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833–1852. [Google Scholar]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Raafat, D.; Von-Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, L.J.; Alakomi, H.L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.M.; Puupponen-Pimiä, R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr Cancer 2006, 54, 18–32. [Google Scholar]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.L.; Wu, J.G.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Kong, M.; Chen, X.G.; Liu, C.S.; Liu, C.G.; Meng, X.H. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloids Surf. B Biointerfaces 2008, 65, 197–202. [Google Scholar] [CrossRef]
- Limam, Z.; Selmi, S.; Sadok, S.; Abed, A.E. Extraction and characterization of chitin and chitosan from crustacean by-products: Biological and physicochemical properties. Afr. J. Biotechnol. 2011, 10, 640–647. [Google Scholar]
- Goy, R.C.; Britto, D.D.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, Y.; Chen, W.; Meng, X.; Liu, B. Hydrophobicity and physicochemical properties of agarose film as affected by chitosan addition. Int. J. Biol. Macromol. 2018, 106, 1307–1313. [Google Scholar]
- El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Preparation and characterization of chitosan/agar blended films: Part 1. Chemical structure and morphology. J. Chem. 2012, 9, 1431–1439. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT-Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Fransiska, D.; Wahyuni, T.; Irianto, H.; Priambudi, P.; Abdullah, A.; Nissa, R.; Firdiana, B. Physical properties of bioplastic agar/chitosan blend. Conf. Ser. Earth Environ. Sci. 2022, 978, 012046. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Songtipya, P. Characteristics of film based on protein isolate from red tilapia muscle with negligible yellow discoloration. Int. J. Biol. Macromol. 2011, 48, 758–767. [Google Scholar] [CrossRef]
- Yang, J.; Kwon, G.J.; Hwang, K.; Kim, D.Y. Cellulose–chitosan antibacterial composite films prepared from LiBr solution. Polymers 2018, 10, 1058. [Google Scholar] [CrossRef]
- Garcia, M.A.; Pinotti, A.; Zaritzky, N.E. Physicochemical, water vapor barrier and mechanical properties of corn starch and chitosan composite films. Starch 2006, 58, 453–463. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P.; Nuthong, P. Film forming ability of gelatins from splendid squid (Loligo formosana) skin bleached with hydrogen peroxide. Food Chem. 2013, 138, 1101–1108. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Shamsuri, A.; Daik, R. Utilization of an ionic liquid/urea mixture as a physical coupling agent for agarose/talc composite films. Materials 2013, 6, 682–698. [Google Scholar] [CrossRef] [Green Version]



| Parameters | Shell | CT | CS−2 | CS−3 | CS−4 |
|---|---|---|---|---|---|
| Yield (%) | - | 20.48 ± 0.72 a | 15.79 ± 0.19 b | 15.62 ± 0.25 b | 14.13 ± 0.09 c |
| Appearance |  |  |  |  |  |
| Color | |||||
| L* | 77.95 ± 0.07 d | 78.03 ± 0.40 d | 78.60 ± 0.24 c | 79.79 ± 0.48 b | 81.86 ± 0.09 a |
| a* | 5.25 ± 0.08 b | 9.69 ± 0.42 a | 5.63 ± 0.49 b | 3.03 ± 0.30 c | 3.45 ± 0.20 c |
| b* | 18.02 ± 0.34 a | 10.56 ± 0.20 d | 14.53 ± 0.47 b | 14.68 ± 0.68 b | 12.38 ± 0.42 c |
| Whiteness (%) | 71.04 ± 0.16 d | 73.76 ± 0.27 c | 73.53 ± 0.21 c | 74.83 ± 0.34 b | 77.76 ± 0.18 a |
| Properties | |||||
| DDA (%) | - | - | 73.56 ± 0.09 c | 74.83 ± 0.18 b | 75.56 ± 0.01 a |
| [η] (dL/g) | - | - | 3.58 ± 0.09 a | 3.44 ± 0.34 a | 2.97 ± 0.16 b |
| Mν (×106 Da) | - | - | 1.44 ± 0.05 a | 1.37 ± 0.18 a | 1.12 ± 0.08 b |
| Type of Bacteria | Pathogenic Bacteria | Concentrations of CS (mg/mL) | Growth Inhibition (%) | ||
|---|---|---|---|---|---|
| CS−2 | CS−3 | CS−4 | |||
| Gram+ | B. cereus | 50.00 | 24.90 ± 2.60 bB | 36.68 ± 3.51 aB | 37.95 ± 3.08 aB |
| 25.00 | 16.41 ± 3.26 bB | 26.79 ± 4.93 aB | 29.27 ± 3.01 aB | ||
| 12.50 | 7.94 ± 3.13 bB | 13.78 ± 3.68 abB | 17.02 ± 2.88 aB | ||
| 6.25 | 1.66 ± 1.89 bA | 3.03 ± 2.22 bB | 8.50 ± 1.74 aB | ||
| 3.13 | - | - | 6.71 ± 0.75 aB | ||
| 1.56 | - | - | - | ||
| S. aureus | 50.00 | 40.21 ± 4.17 bA | 52.81 ± 5.17 aA | 55.90 ± 2.58 aA | |
| 25.00 | 37.98 ± 3.37 bA | 46.22 ± 3.63 aA | 46.32 ± 2.10 aA | ||
| 12.50 | 27.03 ± 4.03 bA | 28.70 ± 2.73 bA | 38.99 ± 1.70 aA | ||
| 6.25 | - | 6.62 ± 1.34 bAB | 35.01 ± 0.87 aA | ||
| 3.13 | - | 0.50 ± 0.38 bA | 13.20 ± 0.77 aA | ||
| 1.56 | - | - | - | ||
| Gram− | E. coli | 50.00 | 31.42 ± 3.33 aB | 38.44 ± 5.59 aB | 38.54 ± 1.95 aB |
| 25.00 | 13.14 ± 3.44 aB | 13.26 ± 5.97 aC | 16.85 ± 3.78 aC | ||
| 12.50 | 11.25 ± 3.22 aB | 11.04 ± 4.41 aB | 15.25 ± 3.25 aB | ||
| 6.25 | - | 9.55 ± 4.22 aA | 12.29 ± 5.65 aB | ||
| 3.13 | - | - | - | ||
| 1.56 | - | - | - | ||
| S. typhimurium | 50.00 | 30.12 ± 7.04 aB | 30.66 ± 4.04 aB | 32.40 ± 5.24 aB | |
| 25.00 | 15.11 ± 6.00 aB | 17.93 ± 6.67 aBC | 21.34 ± 6.18 aC | ||
| 12.50 | 8.81 ± 6.26 aB | 11.73 ± 4.14 aB | 17.66 ± 5.12 aB | ||
| 6.25 | - | 7.27 ± 1.80 aAB | 8.58 ± 2.23 aB | ||
| 3.13 | - | - | - | ||
| 1.56 | - | - | - | ||
| Composite Films (CS−3/AG) | Thickness (mm) | TS (MPa) | EAB (%) | WVP (×10−7 g m m−2 s−1 Pa−1) |
|---|---|---|---|---|
| 100/0 | 0.056 ± 0.012 c | 29.96 ± 1.80 d | 36.52 ± 1.12 a | 3.56 ± 0.10 a |
| 75/25 | 0.044 ± 0.003 b | 38.31 ± 1.13 c | 29.09 ± 3.99 c | 2.40 ± 0.41 b |
| 50/50 | 0.040 ± 0.003 b | 51.69 ± 1.25 b | 32.58 ± 1.81 b | 2.51 ± 0.06 b |
| 25/75 | 0.037 ± 0.003 b | 54.06 ± 3.60 b | 25.27 ± 1.23 d | 2.47 ± 0.08 b |
| 0/100 | 0.024 ± 0.001 a | 89.70 ± 5.08 a | 25.32 ± 1.73 d | 1.55 ± 0.02 c |
| Composite Films (CS−3/AG) | Color Attributes | Appearance | Transparency | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| 100/0 | 87.74 ± 0.69 c | −1.32 ± 0.02 b | 3.09 ± 0.20 a |  | 3.18 ± 0.005 e |
| 75/25 | 88.51 ± 0.04 b | −1.39 ± 0.01 c | 2.28 ± 0.10 b |  | 3.29 ± 0.005 d |
| 50/50 | 88.73 ± 0.07 b | −1.42 ± 0.01 d | 1.61 ± 0.18 c |  | 3.34 ± 0.002 c |
| 25/75 | 89.06 ± 0.03 a | −1.44 ± 0.01 e | 1.02 ± 0.01 d |  | 3.38 ± 0.002 b |
| 0/100 | 89.30 ± 0.21 a | −1.2 ± 0.03 a | −0.19 ± 0.03 e |  | 3.58 ± 0.002 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarnpakdee, S.; Kaewprachu, P.; Jaisan, C.; Senphan, T.; Nagarajan, M.; Wangtueai, S. Extraction and Physico–Chemical Characterization of Chitosan from Mantis Shrimp (Oratosquilla nepa) Shell and the Development of Bio-Composite Film with Agarose. Polymers 2022, 14, 3983. https://doi.org/10.3390/polym14193983
Yarnpakdee S, Kaewprachu P, Jaisan C, Senphan T, Nagarajan M, Wangtueai S. Extraction and Physico–Chemical Characterization of Chitosan from Mantis Shrimp (Oratosquilla nepa) Shell and the Development of Bio-Composite Film with Agarose. Polymers. 2022; 14(19):3983. https://doi.org/10.3390/polym14193983
Chicago/Turabian StyleYarnpakdee, Suthasinee, Pimonpan Kaewprachu, Chalalai Jaisan, Theeraphol Senphan, Muralidharan Nagarajan, and Sutee Wangtueai. 2022. "Extraction and Physico–Chemical Characterization of Chitosan from Mantis Shrimp (Oratosquilla nepa) Shell and the Development of Bio-Composite Film with Agarose" Polymers 14, no. 19: 3983. https://doi.org/10.3390/polym14193983