N-Doped Biochar from Lignocellulosic Biomass for Preparation of Adsorbent: Characterization, Kinetics and Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
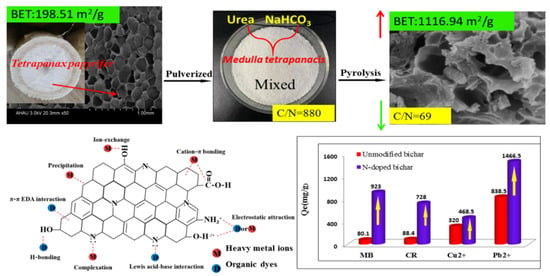
2.2. Preparation of N-Doped Biochars
2.3. Characterization of Biochar
2.4. Adsorption Experiments
2.5. Competitive Adsorption of Multi-Metal
2.6. Regeneration Experiments
3. Results and Discussion
3.1. Characterization of Biochars
3.2. The Influencing Factors on the Adsorption of Biochar
3.2.1. The Influence of pH
3.2.2. The Influence of Time and Adsorption Kinetics
3.2.3. The Influence of Concentration and Adsorption Isotherms
3.3. Adsorption Thermodynamics
3.4. Competitive Adsorption of Multi-Metal
3.5. Comparison with Adsorption Capacity of Other Biopolymer Adsorbents
3.6. Regeneration Study
3.7. Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, H.; Lee, C.; Ong, P.; Wong, K.; Bong, C.; Li, C.; Gao, Y. A review on the comparison between slow pyrolysis and fast pyrolysis on the quality of lignocellulosic and lignin-based biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Gao, L.; Deng, J.; Huang, G.; Li, K.; Cai, K.; Liu, Y.; Huang, F. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 2019, 272, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.D.; Patel, A.K.; Puri, M. Global status of lignocellulosic biorefinery: Challenges and perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Restrepo, Y.A.; Orrego, C.E. Immobilization of enzymes and cells on lignocellulosic materials. Environ. Chem. Lett. 2020, 18, 787–806. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Poornima Devi, T.; Sivashanmugam, P.; Kavitha, S.; Yukesh Kannah, R.; Varjanid, S.; AdishKumar, S.; Kumar, G.; Rajesh Banu, J. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar]
- Pal, U.; Sandoval, A.; Madrid, S.I.U.; Corro, G.; Sharma, V.; Mohanty, P. Mixed titanium, silicon, and aluminum oxide nanostructures as novel adsorbent for removal of rhodamine 6G and methylene blue as cationic dyes from aqueous solution. Chemosphere 2016, 163, 142–152. [Google Scholar] [CrossRef]
- Xu, J.L.; Gu, L.H.; Wang, Z.T.; Bligh, A.; Han, Z.; Liu, S.J. Seventeen steroids from the pith of Tetrapanax papyriferus. J. Asian Nat. Prod. Res. 2016, 12, 1131–1137. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, L.P.; Guan, Y.; Tong, Z.H.; Chen, X.; He, G.Q.; Gao, H. A slow pyrolysis biochar derived from Tetrapanax papyriferum Petiole as an Effective Sorbent for Removing Copper ions from Aqueous Solution. Bioresources 2019, 14, 4430–4453. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, C.Y.; Zhang, X.; Li, J.N.; Wang, F.H.; Zhang, Y.H. Determination of caffeic acid using a glassy carbon electrode modified with porous carbon material obtained from Tetrapanax papyriferus. Ionics 2022, 28, 1441–1450. [Google Scholar] [CrossRef]
- Zhang, L.P.; Li, W.Q.; Cao, H.; Hu, D.; Chen, X.; Guan, Y.; Tang, J.; Gao, H. Ultra-efficient sorption of Cu2+ and Pb2+ ions by light biochar derived from Medulla tetrapanacis. Bioresour. Technol. 2019, 29, 121818. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Eldesoky, G.; Al-Saeedi, S.; Nafady, A.; Al-Kadhi, N.; Al-Muhtaseb, A.; Khan, A.A.; Khan, A. Effective and fast adsorptive removal of toxic cationic dye (MB) from aqueous medium using amino-functionalized magnetic multiwall carbon nanotubes. J. Mol. Liq. 2019, 282, 154–161. [Google Scholar] [CrossRef]
- Benis, K.Z.; Damuchali, A.M.; Soltan, J.; Mcphedran, K.N. Treatment of aqueous arsenic—A review of biochar modification methods. Sci. Total Environ. 2020, 739, 111–126. [Google Scholar]
- Cheng, H.; Zhang, J.; Chen, Y.; Zhang, W.; Ji, R.; Song, Y.; Li, W.; Bian, Y.; Jiang, X.; Xue, J.; et al. Hierarchical porous biochars with controlled pore structures derived from co-pyrolysis of potassium/calcium carbonate with cotton straw for efficient sorption of diethyl phthalate from aqueous solution. Bioresour. Technol. 2022, 346, 126604. [Google Scholar] [CrossRef]
- Arrigo, R.; Jagdale, P.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Structure–Property Relationships in Polyethylene-Based Composites Filled with Biochar Derived from Waste Coffee Grounds. Polymers 2019, 11, 1336. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Y.; Luo, J.; Li, T.; Wang, D.; Luo, S.; Crittendenb, J. 3D hierarchical porous-structured biochar aerogel for rapid and efficient phenicol antibiotics removal from water. Chem. Eng. J. 2019, 368, 639–648. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Xin, J.; Sun, P.; Wu, D. Adsorption behavior and mechanism of Cr (VI) by modified biochar derived from Enteromorpha prolifera. Ecotoxicol. Environ. Saf. 2018, 164, 440–447. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.; Ahmed, N.; Mosa, A.; Niazi, N.; Yousaf, B.; Sharma, A.; Sarkar, B.; Cai, Y.; Chang, S. Nickel in soil and water: Sources, biogeochemistry, and remediation using biochar. J. Hazard. Mater. 2021, 419, 126421. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Li, X.; Wu, Z.; Alsaedi, A.; Hayat, T.; Chen, C.; Li, J. Adsorption of 17 β-estradiol from aqueous solutions by a novel hierarchically nitrogen-doped porous carbon. J. Colloid Interface Sci. 2019, 533, 700–708. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Gao, Y.; Li, A. Facile synthesis of nano ZnO/ZnS modified biochar by directly pyrolyzing of zinc contaminated corn stover for Pb (II), Cu (II) and Cr (VI) removals. Waste Manag. 2018, 796, 25637. [Google Scholar] [CrossRef]
- Yang, Q.; Xiao, Z.; Kong, D.; Zhang, T.; Zhi, L. New insight to the role of edges and heteroatoms in nanocarbons for oxygen reduction reaction. Nano Energy 2019, 66, 104096. [Google Scholar] [CrossRef]
- Cao, L.; Dai, P.; Tang, J.; Li, D.; Chen, R.; Liu, D.; Gu, X.; Li, L.; Bando, Y.; Ok, Y.; et al. A spherical superstructure of boron nitride nanosheets derived from boron-contained metal-organic frameworks. J. Am. Chem. Soc. 2020, 142, 8755–8762. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, M.; Yang, D.; Chen, H.; Li, H. Medulla tetrapanacis-derived O/N co-doped porous carbon materials for efficient oxygen reduction electrocatalysts and high-rate supercapacitors. Electrochim. Acta 2018, 272, 88–96. [Google Scholar] [CrossRef]
- Zhu, K.; Bin, Q.; Shen, Y.; Huang, J.; He, D.; Chen, W. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants. Chem. Eng. J. 2020, 402, 126090. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Pawar, R.R.; Lee, S.; Cho, M.H. Binder-free production of 3D N-doped porous carbon cubes for efficient Pb2+ removal through batch and fixed bed adsorption. J. Clean. Prod. 2017, 168, 290–301. [Google Scholar] [CrossRef]
- Yu, W.; Lian, F.; Cui, G.; Liu, Z. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 2018, 193, 8–16. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, S.; Liu, H.; Ma, Y.; Xu, W. Study of the modification mechanism of heavy metal ions adsorbed by biomass-activated carbon doped with a solid nitrogen source. RSC Adv. 2019, 9, 37440–37449. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P.; Hall, S. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Li, Z.; Xing, B.; Ding, Y.; Li, Y.; Wang, S. A high-performance biochar produced from bamboo pyrolysis with in-situ nitrogen doping and activation for adsorption of phenol and methylene blue. Chin. J. Chem. Eng. 2020, 28, 2872–2880. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, L.P.; Hu, D.; Yang, R.; Zhang, J.; Guan, Y.; Lv, F.X.; Gao, H. A mesoporous nanocellulose/sodium alginate/carboxymethyl-chitosan gel beads for efficient adsorption of Cu2+ and Pb2+. Int. J. Biol. Macromol. 2021, 187, 922–930. [Google Scholar] [CrossRef]
- Ahsaine, H.A.; Anfar, Z.; Zbair, M.; Ezahri, M.; Alem, N. Adsorptive removal of methylene blue and crystal violet onto micro-mesoporous Zr3O/activated carbon composite: A joint experimental and statistical modeling considerations. J. Chem. 2018, 2018, 6982014. [Google Scholar] [CrossRef]
- Fei, L.; Cui, G.; Liu, Z.; Lian, D.; Zhang, G.; Xing, B. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J. Environ. Manag. 2016, 176, 61–68. [Google Scholar]
- Mao, C.; Song, Y.; Chen, L.; Ji, J.; Li, J.; Yuan, X.; Yang, Z.; Ayoko, G.A.; Frost, R.L.; Theiss, F. Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena 2019, 175, 339–348. [Google Scholar] [CrossRef]
- Fan, X.; Wang, X.; Cai, Y.; Xie, H.; Han, S.; Hao, C. Functionalized cotton charcoal/chitosan biomass-based hydrogel for capturing Pb2+, Cu2+ and MB. J. Hazard. Mater. 2022, 423, 127191. [Google Scholar] [CrossRef] [PubMed]
- Ariaeenejad, S.; Motamedi, E.; Salekdeh, G.H. Highly efficient removal of dyes from wastewater using nanocellulose from quinoa husk as a carrier for immobilization of laccase. Bioresour. Technol. 2022, 349, 126833. [Google Scholar] [CrossRef]
- Xing, X.Y.; Li, W.; Zhang, J.; Wu, H.; Guan, Y.; Gao, H. TEMPO-oxidized cellulose hydrogel for efficient adsorption of Cu2+ and Pb2+ modified by polyethyleneimine. Cellulose 2021, 28, 7953–7968. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of biochar and modified biochar in heavy metal contaminated soil: A descriptive review. Sustainability 2021, 13, 14014. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Peng, L.; Han, S.; Hao, C.; Jiang, C.; Wang, H.; Fan, X. Effective removal of heavy metals from water using porous lignin-based adsorbents. Chemosphere 2021, 279, 130504. [Google Scholar] [CrossRef]
- Egbosiguba, T.C.; Abdulkareem, A.S.; Kovo, A.S.; Afolabi, E.A.; Tijani, J.O.; Auta, M.; Roos, W.D. Ultrasonic enhanced adsorption of methylene blue onto the optimized surface area of activated carbon: Adsorption isotherm, kinetics and thermodynamics. Chem. Eng. Res. Des. 2020, 153, 315–336. [Google Scholar] [CrossRef]
- Li, S.; Yao, Y.; Zhao, T.; Wang, M.; Wu, F. Biochars preparation from waste sludge and composts under different carbonization conditions and their Pb (II) adsorption behaviors. Water Sci. Technol. 2019, 80, 1063–1075. [Google Scholar] [CrossRef]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X. Production and optimization of bamboo hydrochars for adsorption of Congo red and 2-naphthol. Bioresour. Technol. 2016, 207, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ojo, T.A.; Ojedokun, A.T.; Bello, O.S. Functionalization of powdered walnut shell with orthophosphoric acid for Congo red dye removal. Part. Sci. Technol. 2019, 37, 74–85. [Google Scholar] [CrossRef]
- Ojedokun, A.T.; Bello, O.S. Liquid phase adsorption of Congo red dye on functionalized corn cobs. J. Dispers. Sci. Technol. 2016, 38, 1285–1294. [Google Scholar] [CrossRef]
- Omidi, S.; Kakanejadifard, A. Eco-friendly synthesis of graphene-chitosan composite hydrogel as efficient adsorbent for Congo red. RSC Adv. 2018, 8, 12179–12189. [Google Scholar] [CrossRef]
- Xu, H.; Bao, Y.; Zuo, S.; Chen, P.; Zhu, Y.; Kong, X.; Chen, Y. Enhanced Electrochemical Performance of Biomass Porous Carbon Adsorption Congo Red. J. Electrochem. Soc. 2022, 169, 010514. [Google Scholar] [CrossRef]
- Bian, P.Y.; Liu, Y.X.; Zheng, X.Q.; Shen, W.B. Removal and Mechanism of Cadmium, Lead and Copper in Water by Functional Modification of Silkworm Excrement Biochar. Polymers 2022, 14, 2889. [Google Scholar] [CrossRef]





| BC | UBC1−1 | UBC1−2 | BC | UBC1−1 | UBC1−2 | ||
|---|---|---|---|---|---|---|---|
| C (%) | 61.58 | 69.07 | 68.94 | SBET (m2/g) | 198.51 | 707.56 | 1116.94 |
| O (%) | 36.87 | 19.27 | 16.96 | Average pore volume (cm3/g) | 0.15 | 0.51 | 0.75 |
| N (%) | 0.07 | 9.24 | 11.54 | Micropore volume (cm3/g) | 0.06 | 0.25 | 0.37 |
| H (%) | 1.48 | 2.42 | 2.56 | Mesopore volume (cm3/g) | 0.08 | 0.26 | 0.38 |
| MB | CR | Cu2+ | Pb2+ | |
|---|---|---|---|---|
| Pseudo-first-order | ||||
| Qe (mg/g) | 192.42 | 559.53 | 154.58 | 423.18 |
| K1 (min−1) | 0.02 | 0.01 | 0.02 | 0.03 |
| R2 | 0.89 | 0.96 | 0.87 | 0.94 |
| Pseudo-second-order | ||||
| Qe (mg/g) | 769.23 | 666.67 | 476.19 | 1250.00 |
| K2 (min−1) | 4.57 × 10−4 | 3.94 × 105 | 2.78 × 10−4 | 1.83 × 10−4 |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 |
| Intraparticle diffusion | ||||
| Kid,1 (mg·g−1·min−1/2) | 293.32 | 48.68 | 182.11 | 299.35 |
| C1 | −104.45 | 15.06 | −218.00 | 13.51 |
| R12 | 0.95 | 0.96 | 0.93 | 0.95 |
| Kid,2 (mg·g−1·min−1/2) | 29.32 | 22.72 | 4.82 | 48.25 |
| C2 | 515.40 | 104.40 | 371.03 | 794.61 |
| R22 | 0.94 | 0.92 | 0.91 | 0.96 |
| Kid,3 (mg·g−1·min−1/2) | 0.66 | 5.55 | 2.60 | 2.15 |
| C3 | 734.47 | 464.00 | 406.19 | 1218.70 |
| R32 | 0.93 | 0.92 | 0.91 | 0.91 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | RL2 | KF (mg/g) | n | RF2 | |
| MB | 909.09 | 0.30 | 0.99 | 490.12 | 8.9969 | 0.98 |
| CR | 714.29 | 0.82 | 0.99 | 553.86 | 10.5164 | 0.85 |
| Cu2+ | 526.32 | 0.20 | 0.99 | 395.18 | 10.7887 | 0.60 |
| Pb2+ | 1666.67 | 0.02 | 0.99 | 115.77 | 5.8173 | 0.90 |
| Sample | T (K) | lnKc | ΔGθ (kJ/mol) | ΔHθ (kJ/mol) | ΔSθ (J/mol/K) |
|---|---|---|---|---|---|
| MB | 303 | 2.76 | −6.95 | 9.34 | 27.51 |
| 313 | 3.19 | −5.30 | |||
| 323 | 2.31 | −6.21 | |||
| CR | 303 | 2.29 | −5.77 | 6.73 | 4.58 |
| 313 | 1.32 | −3.43 | |||
| 323 | 1.98 | −5.31 | |||
| Cu2+ | 303 | 1.47 | −3.69 | 9.90 | 33.97 |
| 313 | 0.91 | −2.36 | |||
| 323 | 0.90 | −2.42 | |||
| Pb2+ | 303 | 3.26 | −8.21 | −1.26 | 54.38 |
| 313 | 3.37 | −8.78 | |||
| 323 | 3.56 | −9.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lv, F.; Yang, R.; Zhang, L.; Tao, W.; Liu, G.; Gao, H.; Guan, Y. N-Doped Biochar from Lignocellulosic Biomass for Preparation of Adsorbent: Characterization, Kinetics and Application. Polymers 2022, 14, 3889. https://doi.org/10.3390/polym14183889
Li J, Lv F, Yang R, Zhang L, Tao W, Liu G, Gao H, Guan Y. N-Doped Biochar from Lignocellulosic Biomass for Preparation of Adsorbent: Characterization, Kinetics and Application. Polymers. 2022; 14(18):3889. https://doi.org/10.3390/polym14183889
Chicago/Turabian StyleLi, Jing, Fanxun Lv, Ran Yang, Liping Zhang, Wei Tao, Guotao Liu, Hui Gao, and Ying Guan. 2022. "N-Doped Biochar from Lignocellulosic Biomass for Preparation of Adsorbent: Characterization, Kinetics and Application" Polymers 14, no. 18: 3889. https://doi.org/10.3390/polym14183889