Synthesis of Water-Dispersed Sulfobetaine Methacrylate–Iron Oxide Nanoparticle-Coated Graphene Composite by Free Radical Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
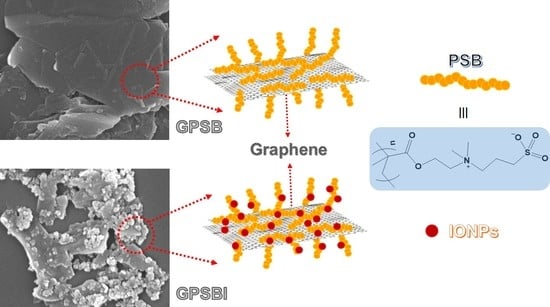
2.3. Graphene-poly[2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium Hydroxide] Composite
2.4. Graphene-poly[2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium Hydroxide]–Iron Oxide Nanoparticle Composite
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Tharkar, P.; Varanasi, R.; Wong, W.S.F.; Jin, C.; Chrzanowski, W. Nano-Enhanced Drug Delivery and Therapeutic Ultrasound for Cancer Treatment and Beyond. Front. Bioeng. Biotechnol. 2019, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Liu, Y.; Xu, J.; Zhu, J. Surface engineering of magnetic iron oxide nanoparticles by polymer grafting: Synthesis progress and biomedical applications. Nanoscale 2020, 12, 14957–14975. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jiang, C.Z.; Roy, V.A.L. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 2016, 8, 19421–19474. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.N.; Chopda, L.V. Application of Iron Oxide Nanomaterials for the Removal of Heavy Metals. J. Nanotechnol. 2014, 2014, 398569. [Google Scholar] [CrossRef]
- Sangaiya, P.; Jayaprakash, R. A Review on Iron Oxide Nanoparticles and Their Biomedical Applications. J. Supercond. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Brammen, M.W.; Zunhammer, F.; Däumler, N.; Fraga-García, P.; Berensmeier, S. Iron Oxide Nanoparticles: Multiwall Carbon Nanotube Composite Materials for Batch or Chromatographic Biomolecule Separation. Nanoscale Res. Lett. 2021, 16, 30. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Kostopoulou, A.; LaGrow, A.P. Magnetic Nanoparticle Composites: Synergistic Effects and Applications. Adv. Sci. 2021, 8, 2004951. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, S.; Uthaman, S.; Huh, K.M.; Koh, Y.S.; Lee, S.; Park, I.-K. Multimodal Composite Iron Oxide Nanoparticles for Biomedical Applications. Tissue Eng. Regen. Med. 2019, 16, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Abdollahi, T.; Motahari, A. Effect of functionalization of iron oxide nanoparticles on the physical properties of poly (aniline-co-pyrrole) based nanocomposites: Experimental and theoretical studies. Arab. J. Chem. 2020, 13, 2331–2339. [Google Scholar] [CrossRef]
- Darabdhara, G.; Borthakur, P.; Das, M.R.; Szunerits, S.; Boukherroub, R. Iron Oxide Nanoparticles-Graphene Composite Materials: Synthesis, Characterization and Applications. In Handbook of Carbon Nano Materials; Springer: Cham, Switzerland, 2020; pp. 265–309. [Google Scholar]
- Rodríguez, B.A.G.; Pérez-Caro, M.; Alencar, R.S.; Filho, A.G.S.; Aguiar, J.A. Graphene nanoribbons and iron oxide nanoparticles composite as a potential candidate in DNA sensing applications. J. Appl. Phys. 2020, 127, 044901. [Google Scholar] [CrossRef]
- Suarez-Martinez, I.; Grobert, N.; Ewels, C.P. Nomenclature of sp2 carbon nanoforms. Carbon 2012, 50, 741–747. [Google Scholar] [CrossRef]
- Sheikhi, M.; Shahab, S.; Balali, E.; Alnajjar, R.; Kaviani, S.; Khancheuski, M.; Al Saud, S. Study of the Ribavirin drug adsorption on the surfaces of carbon nanotube and graphene nanosheet using density functional theory calculations. Bull. Korean Chem. Soc. 2021, 42, 1446–1457. [Google Scholar] [CrossRef]
- Al Faruque, M.A.; Syduzzaman, M.; Sarkar, J.; Bilisik, K.; Naebe, M. A Review on the Production Methods and Applications of Graphene-Based Materials. Nanomaterials 2021, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Hu, J.; Chen, Z.; Zhang, R.; Wang, G.; Kuang, T. Preparation, Properties, and Applications of Graphene-Based Hydrogels. Front. Chem. 2018, 6, 450. [Google Scholar] [CrossRef]
- Velasco, A.; Ryu, Y.K.; Boscá, A.; Ladrón-de-Guevara, A.; Hunt, E.; Zuo, J.; Pedrós, J.; Calle, F.; Martinez, J. Recent trends in graphene supercapacitors: From large area to microsupercapacitors. Sustain. Energy Fuels 2021, 5, 1235–1254. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef]
- Bhol, P.; Yadav, S.; Altaee, A.; Saxena, M.; Misra, P.K.; Samal, A.K. Graphene-Based Membranes for Water and Wastewater Treatment: A Review. ACS Appl. Nano Mater. 2021, 4, 3274–3293. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A. Chapter 15—Graphene-Based Materials for Water Purification. In Nanoscale Materials in Water Purification; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 383–430. [Google Scholar]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Lyubutin, I.S.; Baskakov, A.O.; Starchikov, S.S.; Shih, K.-Y.; Lin, C.-R.; Tseng, Y.-T.; Yang, S.-S.; Han, Z.-Y.; Ogarkova, Y.L.; Nikolaichik, V.I.; et al. Synthesis and characterization of graphene modified by iron oxide nanoparticles. Mater. Chem. Phys. 2018, 219, 411–420. [Google Scholar] [CrossRef]
- Hof, F.; Liu, M.; Valenti, G.; Picheau, E.; Paolucci, F.; Pénicaud, A. Size Control of Nanographene Supported Iron Oxide Nanoparticles Enhances Their Electrocatalytic Performance for the Oxygen Reduction and Oxygen Evolution Reactions. J. Phys. Chem. C 2019, 123, 20774–20780. [Google Scholar] [CrossRef]
- Belaustegui, Y.; Rincón, I.; Fernández-Carretero, F.; Azpiroz, P.; García-Luís, A.; Tanaka, D.A.P. Three-dimensional reduced graphene oxide decorated with iron oxide nanoparticles as efficient active material for high performance capacitive deionization electrodes. Chem. Eng. J. Adv. 2021, 6, 100094. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef]
- Kim, J.D.; Choi, H.C. Enzyme-free H2O2 Sensing at ZnO–Graphene Oxide-modified Glassy Carbon Electrode. Bull. Korean Chem. Soc. 2021, 42, 25–28. [Google Scholar] [CrossRef]
- Umar, A.A.; Patah, M.F.A.; Abnisa, F.; Daud, W.M.A.W. Preparation of magnetized iron oxide grafted on graphene oxide for hyperthermia application. Rev. Chem. Eng. 2020, 38, 569–601. [Google Scholar] [CrossRef]
- Pinto, A.M.; Magalhães, F.D. Graphene-Polymer Composites. Polymers 2021, 13, 685. [Google Scholar] [CrossRef]
- Ganguly, S.; Kanovsky, N.; Das, P.; Gedanken, A.; Margel, S. Photopolymerized Thin Coating of Polypyrrole/Graphene Nanofiber/Iron Oxide onto Nonpolar Plastic for Flexible Electromagnetic Radiation Shielding, Strain Sensing, and Non-Contact Heating Applications. Adv. Mater. Interfaces 2021, 8, 2101255. [Google Scholar] [CrossRef]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Abdel Hamid, Z. Graphene-Based Nanocomposites: Synthesis, Mechanical Properties, and Characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Kurup Sasikala, A.; Thomas, R.G.; Unnithan, A.R.; Saravanakumar, B.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Multifunctional Nanocarpets for Cancer Theranostics: Remotely Controlled Graphene Nanoheaters for Thermo-Chemosensitisation and Magnetic Resonance Imaging. Sci. Rep. 2016, 6, 20543. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, W.; Tao, L.; Li, D.; Boyer, C.A.; Davis, T.P. Thermosensitive graphene nanocomposites formed using pyrene terminal polymers made by RAFT polymerization. J. Polym. Sci. Part A 2010, 48, 425–433. [Google Scholar] [CrossRef]
- Sivakumar, M.P.; Islami, M.; Zarrabi, A.; Khosravi, A.; Peimanfard, S. Polymer-Graphene Nanoassemblies and their Applications in Cancer Theranostics. Anti-Cancer Agents Med. Chem. 2020, 20, 1340–1351. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Q.; Cao, J.; Gao, Y.; Han, S.; Liang, Y.; Zhang, T.; Song, Y.; Sun, Y. Recent progress of graphene oxide-based multifunctional nanomaterials for cancer treatment. Cancer Nanotechnol. 2021, 12, 18. [Google Scholar] [CrossRef]
- Dash, B.S.; Jose, G.; Lu, Y.-J.; Chen, J.-P. Functionalized Reduced Graphene Oxide as a Versatile Tool for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 2989. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-H.; Kim, J.-H. Graphene Oxide–Platinum Nanoparticle Nanocomposites: A Suitable Biocompatible Therapeutic Agent for Prostate Cancer. Polymers 2019, 11, 733. [Google Scholar] [CrossRef]
- Sattari, S.; Adeli, M.; Beyranvand, S. Functionalized Graphene Platforms for Anticancer Drug Delivery. Int. J. Nanomed. 2021, 16, 5955–5980. [Google Scholar] [CrossRef]
- Perumal, S.; Gangadaran, P.; Bae, Y.W.; Ahn, B.-C.; Cheong, I.W. Noncovalent Functionalized Graphene Nanocarriers from Graphite for Treating Thyroid Cancer Cells. ACS Biomater. Sci. Eng. 2021, 7, 2317–2328. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Jebakumar Immanuel Edison, T.N.; Lee, Y.R. Facile synthesis of monodisperse hollow carbon nanospheres using sucrose by carbonization route. Mater. Lett. 2016, 166, 145–149. [Google Scholar] [CrossRef]
- Pinna, N.; Grancharov, S.; Beato, P.; Bonville, P.; Antonietti, M.; Niederberger, M. Magnetite Nanocrystals: Nonaqueous Synthesis, Characterization, and Solubility. Chem. Mater. 2005, 17, 3044–3049. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Wang, S. Enhancing thermoelectric properties of organic composites through hierarchical nanostructures. Sci. Rep. 2013, 3, 3448. [Google Scholar] [CrossRef]
- Çakmak, G.; Öztürk, T. Continuous synthesis of graphite with tunable interlayer distance. Diam. Relat. Mater. 2019, 96, 134–139. [Google Scholar] [CrossRef]
- Girod, M.; Vogel, S.; Szczerba, W.; Thünemann, A.F. How temperature determines formation of maghemite nanoparticles. J. Magn. Magn. Mater. 2015, 380, 163–167. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Syhr, C.; Berensmeier, S. Controlled Synthesis of Magnetic Iron Oxide Nanoparticles: Magnetite or Maghemite? Crystals 2020, 10, 214. [Google Scholar] [CrossRef]
- Karami, H. Synthesis and Characterization of Iron Oxide Nanoparticles by Solid State Chemical Reaction Method. J. Clust. Sci. 2010, 21, 11–20. [Google Scholar] [CrossRef]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef]
- He, K.; Chen, N.; Wang, C.; Wei, L.; Chen, J. Method for Determining Crystal Grain Size by X-Ray Diffraction. Cryst. Res. Technol. 2018, 53, 1700157. [Google Scholar] [CrossRef]
- Kim, S.-G.; Park, O.-K.; Lee, J.H.; Ku, B.-C. Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett. 2013, 14, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Bîru, E.I. Graphene Nanocomposites Studied by Raman Spectroscopy; IntechOpen: London, UK, 2018. [Google Scholar]
- Mohan, V.B.; Bhattacharyya, M.S.; Liu, D.; Jayaraman, K. Improvements in Electronic Structure and Properties of Graphene Derivatives. Adv. Mater. Lett. 2016, 7, 421–429. [Google Scholar] [CrossRef]
- Strankowski, M.; WBodarczyk, D.; Piszczyk, A.; Strankowska, J. Polyurethane Nanocomposites Containing Reduced Graphene Oxide, FTIR, Raman, and XRD Studies. Spectroscopy 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Frank, O.; Mohr, M.; Maultzsch, J.; Thomsen, C.; Riaz, I.; Jalil, R.; Novoselov, K.S.; Tsoukleri, G.; Parthenios, J.; Papagelis, K.; et al. Raman 2D-Band Splitting in Graphene: Theory and Experiment. ACS Nano 2011, 5, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Arunima, R.; Madhulika, S.; Niroj, K.S. Assessing magnetic and inductive thermal properties of various surfactants functionalised Fe3O4 nanoparticles for hyperthermia. Sci. Rep. 2020, 10, 15045. [Google Scholar] [CrossRef]
- Li, S.; Xia, X.; Vogt, B.D. Microwave-Enabled Size Control of Iron Oxide Nanoparticles on Reduced Graphene Oxide. Langmuir 2021, 37, 11131–11141. [Google Scholar] [CrossRef]
- Chien, H.-W.; Lin, H.-Y.; Tsai, C.-Y.; Chen, T.-Y.; Chen, W.-N. Superhydrophilic Coating with Antibacterial and Oil-Repellent Properties via NaIO(4)-Triggered Polydopamine/Sulfobetaine Methacrylate Polymerization. Polymers 2020, 12, 2008. [Google Scholar] [CrossRef]
- Yang, W.J.; Neoh, K.-G.; Kang, E.-T.; Lay-Ming Teo, S.; Rittschof, D. Stainless steel surfaces with thiol-terminated hyperbranched polymers for functionalization via thiol-based chemistry. Polym. Chem. 2013, 4, 3105–3115. [Google Scholar] [CrossRef]
- Hildebrand, V.; Laschewsky, A.; Päch, M.; Müller-Buschbaum, P.; Papadakis, C.M. Effect of the zwitterion structure on the thermo-responsive behaviour of poly(sulfobetaine methacrylates). Polym. Chem. 2017, 8, 310–322. [Google Scholar] [CrossRef]
- Galin, M.; Marchal, E.; Mathis, A.; Meurer, B.; Soto, Y.M.M.; Galin, J.C. Poly(sulphopropylbetaines): 3. Bulk properties. Polymer 1987, 28, 1937–1944. [Google Scholar] [CrossRef]
- Schönemann, E.; Laschewsky, A.; Wischerhoff, E.; Koc, J.; Rosenhahn, A. Surface Modification by Polyzwitterions of the Sulfabetaine-Type, and Their Resistance to Biofouling. Polymers 2019, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Ibrahim, G.P.S.; Isloor, A.M.; Ismail, A.F.; Farnood, R. One-step synthesis of zwitterionic graphene oxide nanohybrid: Application to polysulfone tight ultrafiltration hollow fiber membrane. Sci. Rep. 2020, 10, 6880. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, S.; Atchudan, R.; Lee, Y.R. Synthesis of Water-Dispersed Sulfobetaine Methacrylate–Iron Oxide Nanoparticle-Coated Graphene Composite by Free Radical Polymerization. Polymers 2022, 14, 3885. https://doi.org/10.3390/polym14183885
Perumal S, Atchudan R, Lee YR. Synthesis of Water-Dispersed Sulfobetaine Methacrylate–Iron Oxide Nanoparticle-Coated Graphene Composite by Free Radical Polymerization. Polymers. 2022; 14(18):3885. https://doi.org/10.3390/polym14183885
Chicago/Turabian StylePerumal, Suguna, Raji Atchudan, and Yong Rok Lee. 2022. "Synthesis of Water-Dispersed Sulfobetaine Methacrylate–Iron Oxide Nanoparticle-Coated Graphene Composite by Free Radical Polymerization" Polymers 14, no. 18: 3885. https://doi.org/10.3390/polym14183885