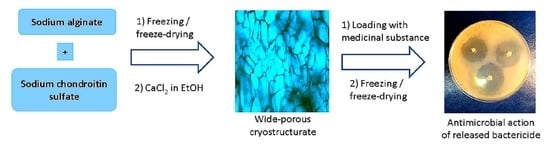
Cryostructuring of Polymeric Systems: 62 Preparation and Characterization of Alginate/Chondroitin Sulfate Cryostructurates Loaded with Antimicrobial Substances †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Complex Ca(ALG-CNS) Cryostructurates
- A.
- The solutions of the same (30 mg/mL) concentration of Na(ALG) and variable (3, 7.5, 15 and 30 mg/mL) concentrations of Na(CNS). In such cases, the mass ratio of these polymers was varied from 1:0.1 to 1:1.
- B.
- The solutions of the same (45 mg/mL) total concentration of both polysaccharides and variable Na(ALG)/Na(CNS) mass ratios (from 1:0.1 to 1:1).
2.3. Loading of Ca(ALG/CNS) Cryostructurates with GMS or DON
2.4. Characterization
2.4.1. Characterization of Dry Ca(ALG/CNS) Cryostructurates
2.4.2. Optical Microscopy
2.4.3. Scanning Electron Microscopy
2.4.4. FTIR-Spectroscopy
2.4.5. Swelling Degree of Ca(ALG/CNS) Cryostructurates in Water
2.4.6. Kinetic Curves for the Drug Release
2.4.7. Characterization of Antibacterial Activity
3. Results and Discussion
3.1. Ca (ALG/CNS) Cryostructurates
3.2. FTIR-Spectra of the Ca(ALG/CNS) Cryostructurates
3.3. Ca(ALG/CNS Cryostructurates Loaded with GMS or DON
3.4. Features of GMS and DON Release from the Drug-Loaded Ca(ALG/CNS) Cryostructurates
3.5. Antibacterial Properties of the GMS- or DON-Loaded Ca(ALG/CNS) Cryostructurates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Siraj, E.A. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Health 2021, 14, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Shtilman, M.I. Polymeric Biomaterials. Part. 1; VSP: Tokyo, Japan, 2003; 293p, ISBN 10:9067643890. [Google Scholar]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Savina, I.; Zoughaib, M.; Yergeshov, A. Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Jagur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Park, H.; Lee, K.-Y. Alginate Hydrogels as Matrices for Tissue Engineering. In Natural-Based Polymers for Biomedical Applications; Reis, R.L., Neves, N.M., Mano, J.F., Gomes, M.E., Marques, A.P., Azevedo, H.S., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 515–532. [Google Scholar] [CrossRef]
- Draget, K.I. Alginates. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 807–829. ISBN 978-1-84569-414-2. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Jiao, G.; Kermanshahi-Pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Polysaccharides constructed hydrogels as vehicles for proteins and peptides. A review. Carbohydr. Polym. 2019, 225, 115210. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. An Up-to-Date Review of Biomaterials Application in Wound Management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell Delivery Systems Using Alginate–Carrageenan Hydrogel Beads and Fibers for Regenerative Medicine Applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef] [PubMed]
- Ghidoni, I.; Chlapanidas, T.; Bucco, M.; Crovato, F.; Marazzi, M.; Vigo, D.; Torre, M.L.; Faustini, M. Alginate cell encapsulation: New advances in reproduction and cartilage regenerative medicine. Cytotechnology 2008, 58, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Santinon, C.; de Freitas, E.D.; da Silva, M.G.C.; Vieira, M.G.A. Modification of valsartan drug release by incorporation into sericin/alginate blend using experimental design methodology. Eur. Polym. J. 2021, 153, 110506. [Google Scholar] [CrossRef]
- Suhail, M.; Li, X.-R.; Liu, J.-Y.; Hsieh, W.-C.; Lin, Y.-W.; Wu, P.-C. Fabrication of alginate based microgels for drug-sustained release: In-vitro and in-vivo evaluation. Int. J. Biol. Macromol. 2021, 192, 958–966. [Google Scholar] [CrossRef]
- Siboro, S.A.; Anugrah, D.S.; Ramesh, K.; Park, S.-H.; Kim, H.-R.; Lim, K.T. Tunable porosity of covalently crosslinked alginate-based hydrogels and its significance in drug release behavior. Carbohydr. Polym. 2021, 260, 117779. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Non-Conventional Methods for Gelation of Alginate. Gels 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Joukhdar, H.; Seifert, A.; Jüngst, T.; Groll, J.; Lord, M.S.; Rnjak-Kovacina, J. Ice Templating Soft Matter: Fundamental Principles and Fabrication Approaches to Tailor Pore Structure and Morphology and Their Biomedical Applications. Adv. Mater. 2021, 33, 2100091. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef]
- Sharma, R.; Kuche, K.; Thakor, P.; Bhavana, V.; Srivastava, S.; Mehra, N.K.; Jain, S. Chondroitin Sulfate: Emerging biomaterial for biopharmaceutical purpose and tissue engineering. Carbohydr. Polym. 2022, 286, 119305. [Google Scholar] [CrossRef] [PubMed]
- Volpi, N. Chondroitin Sulfate Safety and Quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://en.wikipedia.org/wiki/Gentamicin (accessed on 8 August 2022).
- Elina, A.S.; Musatova, I.S.; Peresleni, E.M.; Padeiskaya, E.N. Synthesis, structure, and biological properties of N-oxides of some 2-substituted quinoxalines and pyrazines. Chem. Heterocycl. Compd. 1976, 12, 239–244. [Google Scholar] [CrossRef]
- Yuryev, V.P.; Grinberg, N.V.; Braudo, E.E.; Tolstoguzov, S.V.B. A Study of the Boundary Conditions for the Gel Formation of Alginates of Polyvalent Metals. Starch 1979, 31, 121–124. [Google Scholar] [CrossRef]
- Zvukova, N.D.; Klimova, T.P.; Ivanov, R.V.; Ryabev, A.N.; Tsiskarashvili, A.V.; Lozinsky, V.I. Cryostructuring of Polymeric Systems. 52. Properties, Microstructure and an Example of a Potential Biomedical Use of the Wide-Pore Alginate Cryostructurates. Gels 2019, 5, 25. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Nuzhdina, A.V.; Zvukova, N.D.; Shabatin, V.P.; Semenov, A.M.; Lozinskii, V.I.; Mel’Nikov, M.Y. Hybrid Nanosystems Based on an Antibacterial Preparation of Dioxydine and Metal Nanoparticles (Ag and Cu) Included in Biopolymer Cryostructures. Nanotechnol. Russ. 2018, 13, 182–188. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Karlova, D.L.; Nuzhdina, A.V.; Shabatin, V.P.; Semenov, A.M.; Lozinskii, V.I.; Mel’Nikov, M.Y. Hybrid Systems of Delivery of Long-Acting Drugs Based on Gentamicin Sulfate, Silver, and Copper Nanoparticles, and Gelatin Biopolymer Matrices. Nanotechnol. Russ. 2018, 13, 546–550. [Google Scholar] [CrossRef]
- Scholz, E. Karl-Fischer-Titration. Methoden zur Wasserbestimmung; Springer: Berlin, Germany, 1984; 136p, ISBN 3-540-12846-8. [Google Scholar]
- Lozinsky, V.I.; Shchekoltsova, A.O.; Sinitskaya, E.S.; Vernaya, O.I.; Nuzhdina, A.V.; Bakeeva, I.V.; Ezernitskaya, M.G.; Semenov, A.M.; Shabatina, T.I.; Melnikov, M.Y. Influence of succinylation of a wide-pore albumin cryogels on their properties, structure, biodegradability, and release dynamics of dioxidine loaded in such spongy carriers. Int. J. Biol. Macromol. 2020, 160, 583–592. [Google Scholar] [CrossRef]
- Shao, G.; Hanaor, D.A.H.; Shen, X.; Gurlo, A. Freeze Casting: From Low-Dimensional Building Blocks to Aligned Porous Structures—A Review of Novel Materials, Methods, and Applications. Adv. Mater. 2020, 32, e1907176. [Google Scholar] [CrossRef]
- Saylan, Y.; Denizli, A. Supermacroporous Composite Cryogels in Biomedical Applications. Gels 2019, 5, 20. [Google Scholar] [CrossRef]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; Salas-de la Cruz, D.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, B.; Rao, P.S.; Kasoju, N.; Nagarjuna, V.; Baadhe, R.R. (Eds.) Biomaterials in Tissue Engineering and Regenerative Medicine. From Basic Concepts to State of the Art Approaches; Springer Nature: Singapore, 2021; 1039p, ISBN 13-978-9811600012. [Google Scholar]
- Ramdhani, D. Formulation and Characterization of Chondroitin Sulfate Nanoparticle with Chitosan as Polymer and Kappa Carrageenan as Crosslinker Using the Ionic Gelation Method. J. Nanomed. Nanotechnol. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Utekhina, A.Y.; Sergeev, G.B. Organic nanoparticles. Russ. Chem. Revs. 2011, 80, 219–233. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef]
- Shabatina, T.; Morosov, Y.; Soloviev, A.; Shabatin, A.; Vernaya, O.; Melnikov, M. Cryochemical Production of Drug Nanoforms: Particle Size and Crystal Phase Control of the Antibacterial Medication 2,3-Quinoxalinedimethanol-1,4-dioxide (Dioxidine). Nanomaterials 2021, 11, 1588. [Google Scholar] [CrossRef]
- Yu, L.X.; Furness, M.S.; Raw, A.; Outlaw, K.P.W.; Nashed, N.E.; Ramos, E.; Miller, S.P.F.; Adams, R.C.; Fang, F.; Patel, R.M.; et al. Scientific considerations of pharmaceutical solid polymorphism in abbreviated new drug applications. Pharm. Res. 2003, 20, 531–536. [Google Scholar] [CrossRef]













| Sample | Group | Composition of the Initial Solutions | |||
|---|---|---|---|---|---|
| Na(ALG) Concentration (mg/mL) | Na(CNS) Concentration (mg/mL) | Total Concentration Na(ALG) and Na(CNS) (mg/mL) | Mass Ratio Na(ALG)/Na(CNS) | ||
| 1 | A | 30 | 3 | 33 | 1:0.1 |
| 2 | 30 | 7.5 | 37.5 | 1:0.25 | |
| 3 | 30 | 15 | 45 | 1:0.5 | |
| 4 | 30 | 30 | 60 | 1:1 | |
| 5 | B | 40.9 | 4.1 | 45 | 1:0.1 |
| 6 | 36 | 9 | 45 | 1:0.25 | |
| 7 (3) | 30 | 15 | 45 | 1:0.5 | |
| 8 | 22.5 | 22.5 | 45 | 1:1 | |
| 9 | - | 30 | - | 30 | 1:0 |
| Sample | Group | Disk Diameter (mm) | Disk Thickness (mm) | Disk Weight * (g) | Disk Density * (g/cm3) | Sulfur Content * (wt%) |
| 1 | A | 35.2 ± 0.1 | 2.40 ± 0.10 | 0.0765 ± 0.0011 | 0.0328 ± 0.0020 | 0.57 ± 0.10 |
| 2 | 35.2 ± 0.1 | 2.20 ± 0.10 | 0.0856 ± 0.0014 | 0.0400 ± 0.0022 | 1.00 ± 0.10 | |
| 3 | 35.4 ± 0.1 | 2.35 ± 0.15 | 0.1023 ± 0.0013 | 0.0452 ± 0.0024 | 1.52 ± 0.14 | |
| 4 | 35.3 ± 0.2 | 2.30 ± 0.10 | 0.1174 ± 0.0016 | 0.0553 ± 0.0015 | 2.91 ± 0.25 | |
| 5 | B | 35.2 ± 0.1 | 2.30 ± 0.10 | 0.1014 ± 0.0030 | 0.0448 ± 0.0034 | 0.50 ± 0.10 |
| 6 | 34.8 ± 0.1 | 2.40 ± 0.10 | 0.1024 ± 0.0032 | 0.0451 ± 0.0031 | 0.91 ± 0.15 | |
| 7 (3) | 35.4 ± 0.1 | 2.35 ± 0.15 | 0.1023 ± 0.0013 | 0.0456 ± 0.0024 | 1.52 ± 0.14 | |
| 8 | 35.2 ± 0.2 | 2.30 ± 0.10 | 0.1041 ± 0.0015 | 0.0465 ± 0.0027 | 2.87 ± 0.25 |
| Sample | Group | Swelling Parameters of Ca(ALG/CNS) Cryostructurates | ||
|---|---|---|---|---|
| Stot (g of Bound + Free Water/g of Dry Polymer) | Mafw (g of Free Water/g of Dry Polymer) | Spol * (g of Bound Water/g of Dry Polymer) | ||
| 1 | A | 69.3 ± 4.9 | 62.5 ± 3.9 | 5.8 ± 0.3 |
| 2 | 59.2 ± 3.6 | 50.6 ± 2.8 | 7.0 ± 0.4 | |
| 3 | 39.7 ± 1.9 | 30.9 ± 1.7 | 7.9 ± 0.5 | |
| 4 | 26.2 ± 1.0 | 16.4 ± 0.8 | 8.9 ± 0.7 | |
| 5 | B | 89.1 ± 6.9 | 81.1 ± 6.3 | 7.0 ± 0.2 |
| 6 | 61.2 ± 5.0 | 52.9 ± 4.7 | 7.3 ± 0.3 | |
| 7 (3) | 39.7 ± 1.9 | 30.7 ± 1.7 | 7.9 ± 0.5 | |
| 8 | 26.5 ± 1.3 | 18.3 ± 0.7 | 8.2 ± 0.4 | |
| Composition of the Carrier a | Loaded Antibacterial Substance | GIZ (mm) |
|---|---|---|
| 1 | GMS | 34.1 ± 1.2 |
| 2 | 33.8 ± 1.2 | |
| 3 | 33.6 ± 1.3 | |
| 4 | 33.2 ± 1.2 | |
| 9 | 35.1 ± 1.5 | |
| 1 | DON | 33.9 ± 1.1 |
| 2 | 33.3 ± 1.6 | |
| 3 | 33.5 ± 1.2 | |
| 4 | 34.0 ± 1.8 | |
| 9 | 33.7 ± 1.7 | |
| 1 | none | 0 |
| 2 | 0 | |
| 3 | 0 | |
| 4 | 0 | |
| 9 | 0 | |
| Filter paper | none | 0 |
| GMS | 35.2 ± 1.6 | |
| DON | 35.4 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernaya, O.I.; Ryabev, A.N.; Shabatina, T.I.; Karlova, D.L.; Shabatin, A.V.; Bulatnikova, L.N.; Semenov, A.M.; Melnikov, M.Y.; Lozinsky, V.I. Cryostructuring of Polymeric Systems: 62 Preparation and Characterization of Alginate/Chondroitin Sulfate Cryostructurates Loaded with Antimicrobial Substances. Polymers 2022, 14, 3271. https://doi.org/10.3390/polym14163271
Vernaya OI, Ryabev AN, Shabatina TI, Karlova DL, Shabatin AV, Bulatnikova LN, Semenov AM, Melnikov MY, Lozinsky VI. Cryostructuring of Polymeric Systems: 62 Preparation and Characterization of Alginate/Chondroitin Sulfate Cryostructurates Loaded with Antimicrobial Substances. Polymers. 2022; 14(16):3271. https://doi.org/10.3390/polym14163271
Chicago/Turabian StyleVernaya, Olga I., Andrey N. Ryabev, Tatyana I. Shabatina, Daria L. Karlova, Andrey V. Shabatin, Lyudmila N. Bulatnikova, Alexander M. Semenov, Mikhail Ya. Melnikov, and Vladimir I. Lozinsky. 2022. "Cryostructuring of Polymeric Systems: 62 Preparation and Characterization of Alginate/Chondroitin Sulfate Cryostructurates Loaded with Antimicrobial Substances" Polymers 14, no. 16: 3271. https://doi.org/10.3390/polym14163271