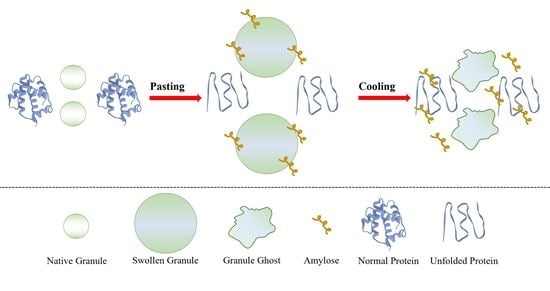
Effects of Mung Bean (Vigna radiata) Protein Isolate on Rheological, Textural, and Structural Properties of Native Corn Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MBPI
2.3. Preparation of NCS/MBPI Blends
2.4. Dynamic Rheological Properties
2.5. Textural Properties
2.6. Morphological Properties
2.7. X–ray Diffraction (XRD) Analysis
2.8. Turbidity Measurements
2.9. Syneresis
2.10. Statistical Analyses
3. Results and Discussion
3.1. Dynamic Rheological Properties
3.2. Textural Properties
3.3. Morphological Properties
3.4. XRD Analysis
3.5. Turbidity Measurements
3.6. Syneresis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, L.; Brennan, M.A.; Mason, S.L.; Zheng, H.; Brennan, C.S. Rheological, pasting and microstructural studies of dairy protein–starch interactions and their application in extrusion-based products: A review. Starch-Stärke 2017, 69, 1600273. [Google Scholar] [CrossRef]
- Bejosano, F.P.; Corke, H. Effect of Amaranthus and buckwheat proteins on the rheological properties of maize starch. Food Chem. 1999, 65, 493–501. [Google Scholar] [CrossRef]
- Zhang, B.; Qiao, D.; Zhao, S.; Lin, Q.; Wang, J.; Xie, F. Starch-based food matrices containing protein: Recent understanding of morphology, structure, and properties. Trends Food Sci. Technol. 2021, 114, 212–231. [Google Scholar] [CrossRef]
- Diyana, Z.; Jumaidin, R.; Selamat, M.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R. Physical properties of thermoplastic starch derived from natural resources and its blends: A review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Tarique, J.; Sapuan, S.; Khalina, A.; Sherwani, S.; Yusuf, J.; Ilyas, R. Recent developments in sustainable arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021, 13, 1191–1219. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Wahab, N.I.A.; Ilyas, R.A. Effect of Kenaf Fibre as Reinforcing Fillers in Corn Starch-Based Biocomposite Film. Polymers 2022, 14, 1590. [Google Scholar] [CrossRef]
- Mohammed, A.A.B.A.; Omran, A.A.B.; Hasan, Z.; Ilyas, R.A.; Sapuan, S.M. Wheat biocomposite extraction, structure, properties and characterization: A review. Polymers 2021, 13, 3624. [Google Scholar] [CrossRef]
- Shams-Abadi, S.T.; Razavi, S.M.A. Cress seed gum improves rheological, textural and physicochemical properties of native wheat starch-sucrose mixture. Int. J. Biol. Macromol. 2021, 181, 945–955. [Google Scholar] [CrossRef]
- Ribotta, P.D.; Colombo, A.; León, A.E.; Añón, M.C. Effects of soy protein on physical and rheological properties of wheat starch. Starch-Stärke 2007, 59, 614–623. [Google Scholar] [CrossRef]
- Ledezma, C.C.Q. Starch interactions with native and added food components. In Starch in Food; Elsevier: Amsterdam, The Netherlands, 2018; pp. 769–801. [Google Scholar]
- Bravo-Núñez, Á.; Garzón, R.; Rosell, C.M.; Gómez, M. Evaluation of starch–protein interactions as a function of pH. Foods 2019, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhong, F.; Goff, H.D.; Li, Y. Study on starch-protein interactions and their effects on physicochemical and digestible properties of the blends. Food Chem. 2019, 280, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Lu, W.; Kelly, A.L.; Zhang, L.; Zheng, B.; Miao, S. Interactions of vegetable proteins with other polymers: Structure-function relationships and applications in the food industry. Trends Food Sci. Technol. 2017, 68, 130–144. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Li, W.; Shu, C.; Yan, S.; Shen, Q. Characteristics of sixteen mung bean cultivars and their protein isolates. Int. J. Food Sci. Technol. 2010, 45, 1205–1211. [Google Scholar] [CrossRef]
- Zhang, D.; Mu, T.; Sun, H. Calorimetric, rheological, and structural properties of potato protein and potato starch composites and gels. Starch-Stärke 2017, 69, 1600329. [Google Scholar] [CrossRef]
- Zheng, M.; Xiao, Y.; Yang, S.; Liu, M.; Feng, L.; Ren, Y.; Yang, X.; Lin, N.; Liu, J. Effect of adding zein, soy protein isolate and whey protein isolate on the physicochemical and in vitro digestion of proso millet starch. Int. J. Food Sci. Technol. 2020, 55, 776–784. [Google Scholar] [CrossRef]
- Joshi, M.; Aldred, P.; Panozzo, J.F.; Kasapis, S.; Adhikari, B. Rheological and microstructural characteristics of lentil starch–lentil protein composite pastes and gels. Food Hydrocoll. 2014, 35, 226–237. [Google Scholar] [CrossRef]
- Sun, Q.; Xiong, C.S.L. Functional and pasting properties of pea starch and peanut protein isolate blends. Carbohydr. Polym. 2014, 101, 1134–1139. [Google Scholar] [CrossRef]
- Joshi, M.; Aldred, P.; Panozzo, J.; Kasapis, S.; Adhikari, B. Rheological properties and microstructure characterization of normal and waxy corn starch dry heated with soy protein isolate. Food Hydrocoll. 2015, 48, 1–7. [Google Scholar]
- El-Adawy, T.A. Functional properties and nutritional quality of acetylated and succinylated mung bean protein isolate. Food Chem. 2000, 70, 83–91. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Hedayati, S.; Niakousari, M. Microstructure, pasting and textural properties of wheat starch-corn starch citrate composites. Food Hydrocoll. 2018, 81, 1–5. [Google Scholar] [CrossRef]
- Singh, N.; Sandhu, K.S.; Kaur, M. Characterization of starches separated from Indian chickpea (Cicer arietinum L.) cultivars. J. Food Hydrocoll. 2004, 63, 441–449. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M. Effects of konjac glucomannan on pasting and rheological properties of corn starch. Food Hydrocoll. 2019, 89, 234–240. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Tang, X. Effects of whey and soy protein addition on bread rheological property of wheat flour. J. Texture Stud. 2018, 49, 38–46. [Google Scholar] [CrossRef]
- Sang, S.; Zhang, H.; Xu, L.; Chen, Y.; Xu, X.; Jin, Z.; Yang, N.; Wu, F.; Li, D. Functionality of ovalbumin during Chinese steamed bread-making processing. Food Chem. 2018, 253, 203–210. [Google Scholar] [CrossRef]
- Niu, H.; Han, Q.; Cao, C.; Liu, Q.; Kong, B. Short-term retrogradation behaviour of corn starch is inhibited by the addition of porcine plasma protein hydrolysates. Int. J. Biol. Macromol. 2018, 115, 393–400. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Fan, Z.; Zhang, X.; Zhong, G. Effect of soybean soluble polysaccharide on the pasting, gels, and rheological properties of kudzu and lotus starches. Food Hydrocoll. 2019, 89, 443–452. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.; Zheng, H.; Brennan, C. The effects of dairy ingredients on the pasting, textural, rheological, freeze-thaw properties and swelling behaviour of oat starch. Food Chem. 2018, 245, 518–524. [Google Scholar] [CrossRef]
- Anbarani, N.M.; Razavi, S.M.A.; Taghizadeh, M. Impact of sage seed gum and whey protein concentrate on the functional properties and retrogradation behavior of native wheat starch gel. Food Hydrocoll. 2021, 111, 106261. [Google Scholar] [CrossRef]
- Liu, J.; Lai, R.; Wang, X.; Wang, H.; Liu, Y. Preparation and Characterization of Composites of Hydroxypropyl Tapioca Starch and Zein. Starch-Stärke 2020, 72, 1900204. [Google Scholar] [CrossRef]
- Niu, L.; Wu, L.; Xiao, J. Inhibition of gelatinized rice starch retrogradation by rice bran protein hydrolysates. Carbohydr. Polym. 2017, 175, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Majzoobi, M.; Ghiasi, F.; Habibi, M.; Hedayati, S.; Farahnaky, A. Influence of soy protein isolate on the quality of batter and sponge cake. J. Food Process. Preserv. 2014, 38, 1164–1170. [Google Scholar] [CrossRef]
- Colombo, A.; León, A.E.; Ribotta, P.D. Rheological and calorimetric properties of corn-, wheat-, and cassava-starches and soybean protein concentrate composites. Starch-Stärke 2011, 63, 83–95. [Google Scholar] [CrossRef]
- Li, J.-Y.; Yeh, A.-I.; Fan, K.-L. Gelation characteristics and morphology of corn starch/soy protein concentrate composites during heating. J. Food Eng. 2007, 78, 1240–1247. [Google Scholar] [CrossRef]
- Ding, Y.; Cheng, J.; Lin, Q.; Wang, Q.; Wang, J.; Yu, G. Effects of endogenous proteins and lipids on structural, thermal, rheological, and pasting properties and digestibility of adlay seed (Coix lacryma-jobi L.) starch. Food Hydrocoll. 2021, 111, 106254. [Google Scholar] [CrossRef]
- Chen, X.; He, X.-W.; Zhang, B.; Fu, X.; Jane, J.-L.; Huang, Q. Effects of adding corn oil and soy protein to corn starch on the physicochemical and digestive properties of the starch. Int. J. Biol. Macromol. 2017, 104, 481–486. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Chen, Y.; Chen, Y. Effect of rice protein on the water mobility, water migration and microstructure of rice starch during retrogradation. Food Hydrocoll. 2019, 91, 136–142. [Google Scholar] [CrossRef]
- Berski, W.; Ziobro, R.; Witczak, M.; Gambuś, H. The retrogradation kinetics of starches of different botanical origin in the presence of glucose syrup. Int. J. Biol. Macromol. 2018, 114, 1288–1294. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016, 61, 843–850. [Google Scholar] [CrossRef]
- Yi-Shen, Z.; Shuai, S.; FitzGerald, R. Mung bean proteins and peptides: Nutritional, functional and bioactive properties. Food Nutr. Res. 2018, 62, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaveh, Z.; Azadmard-Damirchi, S.; Yousefi, G.; Hosseini, S.M.H. A new approach in improving granular cold water swelling starch properties using xanthan gum and β-lactoglobulin/xanthan gum electrostatic coupled gel. Food Hydrocoll. 2021, 113, 106438. [Google Scholar] [CrossRef]
- Hedayati, S.; Shahidi, F.; Koocheki, A.; Farahnaky, A.; Majzoobi, M. Comparing the effects of sucrose and glucose on functional properties of pregelatinized maize starch. Int. J. Biol. Macromol. 2016, 88, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ribotta, P.D.; Rosell, C.M. Effects of enzymatic modification of soybean protein on the pasting and rheological profile of starch–protein systems. Starch-Stärke 2010, 62, 373–383. [Google Scholar] [CrossRef] [Green Version]





| MBPI (%) | Hardness | Cohesiveness | Springiness | Gumminess |
|---|---|---|---|---|
| 0 | 209.61 ± 1.31 a | 0.953 ± 0.013 a | 2.347 ± 0.051 b | 199.81 ± 2.99 a |
| 2 | 201.50 ± 1.41 b | 0.920 ± 0.019 b | 2.478 ± 0.003 a | 185.31 ± 3.97 b |
| 4 | 189.74 ± 2.07 c | 0.887 ± 0.012 c | 2.373 ± 0.004 b | 168.35 ± 4.00 c |
| 6 | 162.79 ± 2.06 d | 0.869 ± 0.012 c | 2.256 ± 0.033 c | 141.40 ± 2.50 d |
| 8 | 150.45 ± 1.91 e | 0.881 ± 0.005 c | 2.326 ± 0.041 b | 132.58 ± 2.40 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarahi, M.; Hedayati, S.; Shahidi, F. Effects of Mung Bean (Vigna radiata) Protein Isolate on Rheological, Textural, and Structural Properties of Native Corn Starch. Polymers 2022, 14, 3012. https://doi.org/10.3390/polym14153012
Tarahi M, Hedayati S, Shahidi F. Effects of Mung Bean (Vigna radiata) Protein Isolate on Rheological, Textural, and Structural Properties of Native Corn Starch. Polymers. 2022; 14(15):3012. https://doi.org/10.3390/polym14153012
Chicago/Turabian StyleTarahi, Mohammad, Sara Hedayati, and Fakhri Shahidi. 2022. "Effects of Mung Bean (Vigna radiata) Protein Isolate on Rheological, Textural, and Structural Properties of Native Corn Starch" Polymers 14, no. 15: 3012. https://doi.org/10.3390/polym14153012