Factors Affecting the Analytical Performance of Magnetic Molecularly Imprinted Polymers
Abstract
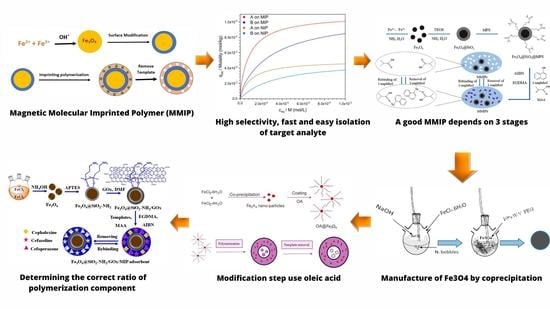
:1. Introduction
2. Synthesis of MMIP
2.1. Magnetic Core-Shell Synthesis
2.1.1. Fe3O4
2.1.2. γ-Fe2O3
2.1.3. Nickel (Ni)
2.1.4. Cobalt (Co)
| Analyte | Magnetic Particle | Magnetisation Saturation | Magnetic Activity | Ref. |
|---|---|---|---|---|
| Di(2-ethylhexyl)phthalate (DEHP) | Fe3O4 | 39.92 emu/g | Superparamagnetic | [56] |
| Resveratrol | Fe3O4 | 53.14 emu/g | Superparamagnetic | [25] |
| Buprenorphine | Fe3O4 | 59.04 emu/g | Supermagnetic | [59] |
| Tadalafil | Fe3O4 | 42 emu/g | Superparamagnetic | [16] |
| Zearalenone | Fe3O4 | 38.10 emu/g | Superparamagnetic | [58] |
| Enantiomer tryptophan (Trp) | Fe3O4 | 69 emu/g | Superparamagnetic | [10] |
| Sulphonamides | Fe3O4-chitosan | 3.91 emu/g | Superparamagnetic | [18] |
| Ivabradine | Fe2O3 | 1.4 emu/g | Low magnetic properties | [64] |
| Chlortoluron | Nickel (II) oxide (NiO) magnetic nanoparticles | 66.7 emu/g | High magnetic activity, ferromagnetism and paramagnetism | [51] |
| Zeolitic Imidazolate Framework-67 (ZIF-67) | Cobalt nanoporous carbon (Co-MNPC) | 34.55 emu/g | High magnetism | [65] |
2.2. MMIP Polymerisation
2.2.1. Free Radical Polymerisation (FRP)
2.2.2. Sol-Gel Polymerisation
| Analyte | Magnetic Particle | Analytical Application | Synthesis Method | Q MMIP (µmol/g) | Q MNIP (µmol/g) | Recovery MMIP (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Chloramphenicol | Fe3O4 magnetite | Honey | Suspension polymerisation | 17.1 | 8.8 | 84.3–90.9 | [8] |
| Resveratrol | Fe3O4@SiO2–MPS nanoparticles | Wine | Surface molecular imprinting | 23.36 | 9.3 | 79.3–90.6 | [25] |
| Tricyclazole | Chitosan Fe3O4 | Rice and water samples | Precipitation Polymerisation | 240.199 | 139.06 | 89.4 (rice), 90.9 (water) | [31] |
| Chloramphenicol | Fe(NO3)3·9H2O | Aquatic environment | Precipitation polymerisation | 71.77, 107.0 and 120.8 at 298, 308 and 318 K | 53.10, 71.44 and 87.14 at 298, 308 and 318 K. | - | [74] |
| Norfloxacin | Fe3O4@SiO2 | lake waste water | sol-gel polymerisation | 1301 | 1121 | 85.4–96.4 | [80] |
| Imidacloprid | Fe3O4 magnetite | Water and apple samples | Suspension polymerisation | 0.094 | 0.039 | 94.0–98.0 | [82] |
MMIP on Drug
MMIP on Macromolecules
MMIP Method Development
3. Factors Affecting MMIP Synthesis
3.1. Factors Affecting the Manufacture of Magnetic Core Particles
3.1.1. Effect of Temperature and Reaction Time in the Manufacture of Magnetite Fe3O4
3.1.2. Effect of pH Value
3.2. Factors Affecting the Magnetic Coating of the Shell Using Modified Components
3.2.1. Effect of Modified Component Types
Oleic Acid
Chitosan
Silica
3.2.2. Effect of Initial Concentration of FeCl3 and the Molar Ratio of Surfactant
3.3. Factors in the Synthesis of MMIP Using Polymerisation Components
4. Conclusions
- New processes for nanomaterials and optimization of the modification procedures in the development of MMIP synthesis;
- Further exploration of surface modification materials, such as chitosan and cyclodextrine or changes to the carboxylate groups and other amines;
- Discovering other magnetic metals besides the existing ones and modifying the magnetic properties of metals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-APTES | Aminopropyltriethoxysilane |
| DMSO | Dimethyl Sulphoxide |
| FM | Ferrimagnetic |
| FRP | Free Radical Polymerisation |
| IF | Imprinting Factor |
| LOD | Limit Of Detection (LOD) |
| LOQ | Limit Of Quantification (LOQ) |
| MIP | Molecular Imprinted Polymer |
| MIT | Molecular Imprinted Technology |
| MMIP | Magnetic Molecularly Imprinted Polymer |
| MMI-SPE | Magnetic Molecular Imprinted Solid Phase Extraction |
| MNIP | Magnetic Non-Imprinted Polymer |
| MNPs | Magnetic Nanoparticles |
| MPS | 3-(Trimethoxysilyl) Propyl Methacrylate |
| MS | Magnetisation Saturation |
| MSPE | Magnetic Solid Phase Extraction |
| MTEOS | Methacryloxypropyltrimethoxysilane |
| NIP | Non-Imprinted Polymer |
| NPs | Nanoparticles |
| PEG | Polyethylene Glycol |
| Q | Maximum Adsorption |
| SDS | Sodium Dodecyl Sulphate |
| SPE | Solid Phase Extraction |
| SPM | Superparamagnetic |
| TEM | Transmission Electron Microscopy |
| TEOS | Tetraethyl Orthosilicate |
| TPA | Terephthalic acid |
| VSM | Vibrating Sample Magnetometer |
| XRD | X-ray Diffraction |
References
- Singh, M.; Singh, S.; Singh, S.P.; Patel, S.S. Recent Advancement of Carbon Nanomaterials Engrained Molecular Imprinted Polymer for Environmental Matrix. Trends Environ. Anal. Chem. 2020, 27, e00092. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Zhu, G. Molecularly Imprinted Porous Aromatic Frameworks for Molecular Recognition. ACS Cent. Sci. 2020, 6, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Miao, Y.; Wei, D.; Zhang, Y.; Zhou, Y. Efficient Removal of Norfloxacin in Water Using Magnetic Molecularly Imprinted Polymer. Chemosphere 2021, 262, 128032. [Google Scholar] [CrossRef] [PubMed]
- Martín-Esteban, A. Molecularly-Imprinted Polymers as a Versatile, Highly Selective Tool in Sample Preparation. TrAC Trends Anal. Chem. 2013, 45, 169–181. [Google Scholar] [CrossRef]
- Adumitrăchioaie, A.; Tertiş, M.; Cernat, A.; Săndulescu, R.; Cristea, C. Electrochemical Methods Based on Molecularly Imprinted Polymers for Drug Detection. A Review. Int. J. Electrochem. Sci. 2018, 13, 2556–2576. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent Advances in Molecular Imprinting Technology: Current Status, Challenges and Highlighted Applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef]
- Ansari, S. Application of Magnetic Molecularly Imprinted Polymer as a Versatile and Highly Selective Tool in Food and Environmental Analysis: Recent Developments and Trends. TrAC Trends Anal. Chem. 2017, 90, 89–106. [Google Scholar] [CrossRef]
- Chen, L.; Li, B. Magnetic Molecularly Imprinted Polymer Extraction of Chloramphenicol from Honey. Food Chem. 2013, 141, 23–28. [Google Scholar] [CrossRef]
- Ji, W.; Sun, R.; Duan, W.; Wang, X.; Wang, T.; Mu, Y.; Guo, L. Selective Solid Phase Extraction of Chloroacetamide Herbicides from Environmental Water Samples by Amphiphilic Magnetic Molecularly Imprinted Polymers. Talanta 2017, 170, 111–118. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Jia, L. Synthesis of Molecularly Imprinted Polymer Modified Magnetic Particles for Chiral Separation of Tryptophan Enantiomers in Aqueous Medium. J. Chromatogr. A 2020, 1622, 461147. [Google Scholar] [CrossRef]
- Xu, L.; Pan, J.; Dai, J.; Li, X.; Hang, H.; Cao, Z.; Yan, Y. Preparation of Thermal-Responsive Magnetic Molecularly Imprinted Polymers for Selective Removal of Antibiotics from Aqueous Solution. J. Hazard. Mater. 2012, 233, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Yu, X. Magnetic Molecularly Imprinted Polymers: Synthesis and Applications in the Selective Extraction of Antibiotics. Front. Chem. 2021, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Karimi, M. Recent Configurations and Progressive Uses of Magnetic Molecularly Imprinted Polymers for Drug Analysis. Talanta 2017, 167, 470–485. [Google Scholar] [CrossRef]
- Kwaśniewska, K.; Gadzała-Kopciuch, R.; Buszewski, B. Magnetic Molecular Imprinted Polymers as a Tool for Isolation and Purification of Biological Samples. Open Chem. 2015, 13, 1228–1235. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, Q.; Bai, S.; Wang, M.; Wang, L. Nanosized Difunctional Photo Responsive Magnetic Imprinting Polymer for Electrochemically Monitored Light-Driven Paracetamol Extraction. ACS Appl. Mater. Interfaces 2017, 9, 44114–44123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, M.J.; Wang, S.; Wang, R.Y.; Wu, X.L.; Wen, T.T.; Yuan, L.H.; Dai, P.; Lin, Y.H.; Zhou, X.M. Preparation of Imprinted Polymers at Surface of Magnetic Nanoparticles for the Selective Extraction of Tadalafil from Medicines. ACS Appl. Mater. Interfaces 2011, 3, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Lamaoui, A.; Lahcen, A.A.; García-Guzmán, J.J.; Palacios-Santander, J.M.; Cubillana-Aguilera, L.; Amine, A. Study of Solvent Effect on the Synthesis of Magnetic Molecularly Imprinted Polymers Based on Ultrasound Probe: Application for Sulfonamide Detection. Ultrason. Sonochem. 2019, 58, 104670. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, C.; Li, X.; Liu, L. Preparation and Application of Epitope Magnetic Molecularly Imprinted Polymers for Enrichment of Sulfonamide Antibiotics in Water. Electrophoresis 2017, 38, 2462–2467. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, L.D.; Shan, Y.B.; Yuan, B.F.; Feng, Y.Q. Preparation of Magnetic Poly(Diethyl Vinylphosphonate-Co-Ethylene Glycol Dimethacrylate) for the Determination of Chlorophenols in Water Samples. J. Chromatogr. A 2012, 1265, 24–30. [Google Scholar] [CrossRef]
- He, H.; Xiao, D.; He, J.; Li, H.; He, H.; Dai, H.; Peng, J. Preparation of a Core-Shell Magnetic Ion-Imprinted Polymer via a Sol-Gel Process for Selective Extraction of Cu(Ii). Analyst 2014, 139, 2459–2466. [Google Scholar] [CrossRef]
- Attallah, O.A.; Al-Ghobashy, M.A.; Ayoub, A.T.; Nebsen, M. Magnetic Molecularly Imprinted Polymer Nanoparticles for Simultaneous Extraction and Determination of 6-Mercaptopurine and Its Active Metabolite Thioguanine in Human Plasma. J. Chromatogr. A 2018, 1561, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, Y.; Funaya, N.; Matsunaga, H.; Haginaka, J. Preparation of Magnetic Molecularly Imprinted Polymers for Bisphenol A and Its Analogues and Their Application to the Assay of Bisphenol A in River Water. J. Pharm. Biomed. Anal. 2013, 75, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Uzuriaga-Sánchez, R.J.; Khan, S.; Wong, A.; Picasso, G.; Pividori, M.I.; Sotomayor, M.D.P.T. Magnetically Separable Polymer (Mag-MIP) for Selective Analysis of Biotin in Food Samples. Food Chem. 2016, 190, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Rajput, Y.S.; Singh, G.; Sharma, R. Synthesis and Characterization of Oxytetracycline Imprinted Magnetic Polymer for Application in Food. Appl. Nanosci. 2016, 6, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.F.; Xie, X.Y.; Shi, Y.P. Preparation of Magnetic Molecularly Imprinted Polymer for Selective Recognition of Resveratrol in Wine. J. Chromatogr. A 2013, 1300, 112–118. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Zhang, Y.; Zhang, Q.; Guo, J.; Huang, W.; Shi, S.; Chen, X. High-Capacity Thermo-Responsive Magnetic Molecularly Imprinted Polymers for Selective Extraction of Curcuminoids. J. Chromatogr. A 2014, 1354, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ariani, M.D.; Zuhrotun, A.; Manesiotis, P.; Hasanah, A.N. Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products. Polymers 2022, 14, 1389. [Google Scholar] [CrossRef]
- Wang, D.D.; Gao, D.; Xu, W.J.; Li, F.; Yin, M.N.; Fu, Q.F.; Xia, Z.N. Magnetic Molecularly Imprinted Polymer for the Selective Extraction of Hesperetin from the Dried Pericarp of Citrus Reticulata Blanco. Talanta 2018, 184, 307–315. [Google Scholar] [CrossRef]
- Xie, X.; Wei, F.; Chen, L.; Wang, S. Preparation of Molecularly Imprinted Polymers Based on Magnetic Nanoparticles for the Selective Extraction of Protocatechuic Acid from Plant Extracts. J. Sep. Sci. 2015, 38, 1046–1052. [Google Scholar] [CrossRef]
- Kubo, T.; Otsuka, K. Recent Progress in Molecularly Imprinted Media by New Preparation Concepts and Methodological Approaches for Selective Separation of Targeting Compounds. TrAC Trends Anal. Chem. 2016, 81, 102–109. [Google Scholar] [CrossRef]
- Laskar, N.; Ghoshal, D.; Gupta, S. Chitosan-Based Magnetic Molecularly Imprinted Polymer: Synthesis and Application in Selective Recognition of Tricyclazole from Rice and Water Samples. Iran. Polym. J. 2021, 30, 121–134. [Google Scholar] [CrossRef]
- Chen, L.; Li, B. Application of Magnetic Molecularly Imprinted Polymers in Analytical Chemistry. Anal. Methods 2012, 4, 2613–2621. [Google Scholar] [CrossRef]
- Gao, G.; Shi, R.; Qin, W.; Shi, Y.; Xu, G.; Qiu, G.; Liu, X. Solvothermal Synthesis and Characterization of Size-Controlled Monodisperse Fe3O4 Nanoparticles. J. Mater. Sci. 2010, 45, 3483–3489. [Google Scholar] [CrossRef]
- Yan, A.; Liu, X.; Qiu, G.; Wu, H.; Yi, R.; Zhang, N.; Xu, J. Solvothermal Synthesis and Characterization of Size-Controlled Fe3O4 Nanoparticles. J. Alloys Compd. 2008, 458, 487–491. [Google Scholar] [CrossRef]
- Faiyas, A.P.A.; Vinod, E.M.; Joseph, J.; Ganesan, R.; Pandey, R.K. Dependence of PH and Surfactant Effect in the Synthesis of Magnetite (Fe3O4) Nanoparticles and Its Properties. J. Magn. Magn. Mater. 2010, 322, 400–404. [Google Scholar] [CrossRef]
- Gao, R.; Kong, X.; Wang, X.; He, X.; Chen, L.; Zhang, Y. Preparation and Characterization of Uniformly Sized Molecularly Imprinted Polymers Functionalized with Core-Shell Magnetic Nanoparticles for the Recognition and Enrichment of Protein. J. Mater. Chem. 2011, 21, 17863–17871. [Google Scholar] [CrossRef]
- Tom, L.A.; Schneck, N.A.; Walter, C. Improving the Imprinting Effect by Optimizing Template:Monomer:Cross-Linker Ratios in a Molecularly Imprinted Polymer for Sulfadimethoxine. J. Chromatogr. B 2012, 909, 61–64. [Google Scholar] [CrossRef]
- Dong, W.; Yan, M.; Liu, Z.; Wu, G.; Li, Y. Effects of Solvents on the Adsorption Selectivity of Molecularly Imprinted Polymers: Molecular Simulation and Experimental Validation. Sep. Purif. Technol. 2007, 53, 183–188. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, W.; Guo, C.; Zhang, J.; Yu, L.; Zhang, G.; Wang, X.; Fang, G.; Sun, D. Magnetic Molecularly Imprinted Electrochemical Sensors: A Review. Anal. Chim. Acta 2020, 1106, 1–21. [Google Scholar] [CrossRef]
- Aguilar-Arteaga, K.; Rodriguez, J.A.; Barrado, E. Magnetic Solids in Analytical Chemistry: A Review. Anal. Chim. Acta 2010, 674, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Poonia, K.; Raizada, P.; Singh, A.; Verma, N.; Ahamad, T.; Alshehri, S.M.; Khan, A.A.P.; Singh, P.; Hussain, C.M. Magnetic Molecularly Imprinted Polymer Photocatalysts: Synthesis, Applications and Future Perspective. J. Ind. Eng. Chem. 2022. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Zheng, J.; Zhu, F.; Xie, L.; Ouyang, G. Synthesis and Application of Magnetic Molecularly Imprinted Polymers in Sample Preparation. Anal. Bioanal. Chem. 2018, 410, 3991–4014. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Lu, Z.; Ge, H.; Liu, X.; Chen, B.; Zou, P.; Wang, X.; He, H.; Zeng, X.; Wang, Y. Electrochemical Creatinine Sensor Based on a Glassy Carbon Electrode Modified with a Molecularly Imprinted Polymer and a Ni@polyaniline Nanocomposite. Microchim. Acta 2017, 184, 261–269. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Z.; Liu, Y.; Meng, M.; Ni, L.; Meng, X.; Zhong, G.; Liu, F.; Gao, Y. Monodisperse Magnetic Ion Imprinted Polymeric Microparticles Prepared by RAFT Polymerization Based on γ-Fe2O3@meso-SiO2 Nanospheres for Selective Solid-Phase Extraction of Cu(Ii) in Water Samples. RSC Adv. 2015, 5, 52369–52381. [Google Scholar] [CrossRef]
- Li, X.; Pan, J.; Dai, J.; Dai, X.; Xu, L.; Wei, X.; Hang, H.; Li, C.; Liu, Y. Surface Molecular Imprinting onto Magnetic Yeast Composites via Atom Transfer Radical Polymerization for Selective Recognition of Cefalexin. Chem. Eng. J. 2012, 198–199, 503–511. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Rahimi, M.; Ahmadi, M.; Soleimani, M. Preconcentration and Spectrophotometric Determination of Oxymetholone in the Presence of Its Main Metabolite (Mestanolone) Using Modified Maghemite Nanoparticles in Urine Sample. Talanta 2013, 115, 468–473. [Google Scholar] [CrossRef]
- Can, M.M.; Coşkun, M.; Firat, T. A Comparative Study of Nanosized Iron Oxide Particles; Magnetite (Fe3O4), Maghemite (γ-Fe2O3) and Hematite (α-Fe2O3), Using Ferromagnetic Resonance. J. Alloys Compd. 2012, 542, 241–247. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Y.; Li, J.J.; Su, X.M.; Wu, Z.Y.; Li, P.F.; Lei, F.H.; Tan, X.C.; Shi, Z.W. Synthesis of Magnetic Molecularly Imprinted Polymers for the Selective Separation and Determination of Metronidazole in Cosmetic Samples. Anal. Bioanal. Chem. 2015, 407, 3875–3880. [Google Scholar] [CrossRef]
- Li, Y.F.; Qiao, L.Q.; Li, F.W.; Ding, Y.; Yang, Z.J.; Wang, M.L. Determination of Multiple Pesticides in Fruits and Vegetables Using a Modified Quick, Easy, Cheap, Effective, Rugged and Safe Method with Magnetic Nanoparticles and Gas Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1361, 77–87. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wei, X.; Li, J. A Sensitive and Renewable Chlortoluron Molecularly Imprinted Polymer Sensor Based on the Gate-Controlled Catalytic Electrooxidation of H2O2 on Magnetic Nano-NiO. Electroanalysis 2013, 25, 1286–1293. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, R.; Zhu, F.; Liu, H.; Ouyang, G. Application of Functionalized Magnetic Nanoparticles in Sample Preparation. Anal. Bioanal. Chem. 2014, 406, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, P.; Isasi, J.; Caballero, A.C.; Marco, J.F.; Martín-Hernández, F. Magnetic and Structural Studies of Fe3O4 Nanoparticles Synthesized via Coprecipitation and Dispersed in Different Surfactants. Ceram. Int. 2017, 43, 10333–10340. [Google Scholar] [CrossRef]
- Hariani, P.L.; Faizal, M.; Ridwan, R.; Marsi, M.; Setiabudidaya, D. Synthesis and Properties of Fe3O4 Nanoparticles by Co-Precipitation Method to Removal Procion Dye. Int. J. Environ. Sci. Dev. 2013, 4, 336–340. [Google Scholar] [CrossRef]
- Shao, M.; Ning, F.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Preparation of Fe3O4@SiO2@layered Double Hydroxide Core-Shell Microspheres for Magnetic Separation of Proteins. J. Am. Chem. Soc. 2012, 134, 1071–1077. [Google Scholar] [CrossRef]
- Ali Zulfikar, M.; Rizqi Utami, A.; Handayani, N.; Wahyuningrum, D.; Setiyanto, H.; Yudhistira Azis, M. Removal of Phthalate Ester Compound from PVC Plastic Samples Using Magnetic Molecularly Imprinted Polymer on the Surface of Superparamagnetic Fe3O4 (Fe3O4@MIPs). Environ. Nanotechnol. Monit. Manag. 2022, 17, 100646. [Google Scholar] [CrossRef]
- Sirivat, A.; Paradee, N. Facile Synthesis of Gelatin-Coated Fe3O4 Nanoparticle: Effect of PH in Single-Step Co-Precipitation for Cancer Drug Loading. Mater. Des. 2019, 181, 107942. [Google Scholar] [CrossRef]
- Fu, H.; Liu, J.; Xu, W.; Wang, H.; Liao, S.; Chen, G. A New Type of Magnetic Molecular Imprinted Material Combined with β-Cyclodextrin for the Selective Adsorption of Zearalenone. J. Mater. Chem. B 2020, 8, 10966–10976. [Google Scholar] [CrossRef]
- Habibi, B.; Rostamkhani, S.; Hamidi, M. Magnetic Molecularly Imprinted Polymer Nanoparticles for Dispersive Micro Solid-Phase Extraction and Determination of Buprenorphine in Human Urine Samples by HPLC-FL. J. Iran. Chem. Soc. 2018, 15, 1569–1580. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Row, K.H. Magnetic Solid-Phase Extraction with Fe3O4/Molecularly Imprinted Polymers Modified by Deep Eutectic Solvents and Ionic Liquids for the Rapid Purification of Alkaloid Isomers (Theobromine and Theophylline) from Green Tea. Molecules 2017, 22, 1061. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Su, L.; Wang, P.; Gao, Y. Rapid and Selective Extraction of Multiple Sulfonamides from Aqueous Samples Based on Fe3O4-Chitosan Molecularly Imprinted Polymers. Anal. Methods 2015, 7, 8704–8713. [Google Scholar] [CrossRef]
- Ferrone, V.; Bruni, P.; Canale, V.; Sbrascini, L.; Nobili, F.; Carlucci, G.; Ferrari, S. Simple Synthesis of Fe3O4@-Activated Carbon from Wastepaper for Dispersive Magnetic Solid-Phase Extraction of Non-Steroidal Anti-Inflammatory Drugs and Their UHPLC–PDA Determination in Human Plasma. Fibers 2022, 10, 58. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.P. Sensors and Biosensors Based on Magnetic Nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; Madbouly, A.; el Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. Molecularly Imprinted Electrochemical Sensor-Based Fe2O3@MWCNTs for Ivabradine Drug Determination in Pharmaceutical Formulation, Serum, and Urine Samples. Front. Bioeng. Biotechnol. 2021, 9, 648704. [Google Scholar] [CrossRef]
- Wu, C.; He, J.; Chen, N.; Li, Y.; Yuan, L.; Zhao, D.; He, L.; Gu, K.; Zhang, S. Synthesis of Cobalt-Based Magnetic Nanoporous Carbon Core-Shell Molecularly Imprinted Polymers for the Solid-Phase Extraction of Phthalate Plasticizers in Edible Oil. Anal. Bioanal. Chem. 2018, 410, 6943–6954. [Google Scholar] [CrossRef]
- Kodama, R.H.; Berkowitz, A.M.; McNiff, E.J., Jr.; Foner, S. Surface Spin Disorder in NiFe2O4 Nanoparticles. Am. Phys. Soc. 1996, 77, 394–397. [Google Scholar]
- Marfà, J.; Pupin, R.R.; Sotomayor, M.; Pividori, M.I. Magnetic-Molecularly Imprinted Polymers in Electrochemical Sensors and Biosensors. Anal. Bioanal. Chem. 2021, 413, 6141–6157. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Yunus, R.M.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Z.; Yan, F.; Sun, B. Extraction of the Toluene Exposure Biomarkers Hippuric Acid and Methylhippuric Acid Using a Magnetic Molecularly Imprinted Polymer, and Their Quantitation by LC-MS/MS. Microchim. Acta 2019, 186, 135. [Google Scholar] [CrossRef]
- Masthoff, I.C.; Kraken, M.; Mauch, D.; Menzel, D.; Munevar, J.A.; Baggio Saitovitch, E.; Litterst, F.J.; Garnweitner, G. Study of the Growth Process of Magnetic Nanoparticles Obtained via the Non-Aqueous Sol-Gel Method. J. Mater. Sci. 2014, 49, 4705–4714. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Q.; Liu, S.; Chen, L. Preparation of a Magnetic Molecularly Imprinted Polymer with Pseudo Template for Rapid Simultaneous Determination of Cyromazine and Melamine in Bio-Matrix Samples. Anal. Bioanal. Chem. 2012, 404, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Hang, H.; Li, C.; Pan, J.; Li, L.; Dai, J.; Dai, X.; Yu, P.; Feng, Y. Selective Separation of Lambdacyhalothrin by Porous/Magnetic Molecularly Imprinted Polymers Prepared by Pickering Emulsion Polymerization. J. Sep. Sci. 2013, 36, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; He, J.; Xie, A.; Gao, L.; Pan, J.; Chen, X.; Zhou, Z.; Wei, X.; Yan, Y. Novel Pitaya-Inspired Well-Defined Core-Shell Nanospheres with Ultrathin Surface Imprinted Nanofilm from Magnetic Mesoporous Nanosilica for Highly Efficient Chloramphenicol Removal. Chem. Eng. J. 2016, 284, 812–822. [Google Scholar] [CrossRef]
- Men, H.F.; Liu, H.Q.; Zhang, Z.L.; Huang, J.; Zhang, J.; Zhai, Y.Y.; Li, L. Synthesis, Properties and Application Research of Atrazine Fe3O4@SiO2 Magnetic Molecularly Imprinted Polymer. Environ. Sci. Pollut. Res. 2012, 19, 2271–2280. [Google Scholar] [CrossRef]
- Ebrahimzadeh, H.; Asgharinezhad, A.A.; Moazzen, E.; Amini, M.M.; Sadeghi, O. A Magnetic Ion-Imprinted Polymer for Lead(II) Determination: A Study on the Adsorption of Lead(II) by Beverages. J. Food Compos. Anal. 2015, 41, 74–80. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, W.; Zhao, H.; Dramou, P.; Pham-Huy, C.; He, J.; He, H. Adsorption Behavior of a Computer-Aid Designed Magnetic Molecularly Imprinted Polymer via Response Surface Methodology. RSC Adv. 2015, 5, 61161–61169. [Google Scholar] [CrossRef]
- Chang, T.; Liu, Y.; Yan, X.; Liu, S.; Zheng, H. One-Pot Synthesis of Uniform and Monodisperse Superparamagnetic Molecularly Imprinted Polymer Nanospheres through a Sol-Gel Process for Selective Recognition of Bisphenol A in Aqueous Media. RSC Adv. 2016, 6, 66297–66306. [Google Scholar] [CrossRef]
- Li, C.; Ma, X.; Zhang, X.; Wang, R.; Li, X.; Liu, Q. Preparation of Magnetic Molecularly Imprinted Polymer Nanoparticles by Surface Imprinting by a Sol–Gel Process for the Selective and Rapid Removal of Di-(2-Ethylhexyl) Phthalate from Aqueous Solution. J. Sep. Sci. 2017, 40, 1621–1628. [Google Scholar] [CrossRef]
- Li, G.; Zha, J.; Niu, M.; Hu, F.; Hui, X.; Tang, T.; Fizir, M.; He, H. Bifunctional Monomer Molecularly Imprinted Sol-Gel Polymers Based on the Surface of Magnetic Halloysite Nanotubes as an Effective Extraction Approach for Norfloxacin. Appl. Clay Sci. 2018, 162, 409–417. [Google Scholar] [CrossRef]
- Deiminiat, B.; Rounaghi, G.H.; Arbab-Zavar, M.H. Development of a New Electrochemical Imprinted Sensor Based on Poly-Pyrrole, Sol–Gel and Multiwall Carbon Nanotubes for Determination of Tramadol. Sens. Actuators B Chem. 2017, 238, 651–659. [Google Scholar] [CrossRef]
- Ilktaç, R.; Gümüs, Z.P. Sensitive and Selective Determination of Imidacloprid with Magnetic Molecularly Imprinted Polymer by Using LC/Q-TOF/MS. Turk. J. Chem. 2021, 45, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular Imprinting: Perspectives and Applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Lofgreen, J.E.; Ozin, G.A. Controlling Morphology and Porosity to Improve Performance of Molecularly Imprinted Sol-Gel Silica. Chem. Soc. Rev. 2014, 43, 911–933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Zhang, C.; Su, X.; Huang, S.; Zhao, M. Enhancing the Selectivity of Enzyme Detection by Using Tailor-Made Nanoparticles. Anal. Chem. 2013, 85, 4853–4857. [Google Scholar] [CrossRef]
- Kan, X.; Zhao, Q.; Shao, D.; Geng, Z.; Wang, Z.; Zhu, J.J. Preparation and Recognition Properties of Bovine Hemoglobin Magnetic Molecularly Imprinted Polymers. J. Phys. Chem. B 2010, 114, 3999–4004. [Google Scholar] [CrossRef]
- Jing, T.; Du, H.; Dai, Q.; Xia, H.; Niu, J.; Hao, Q.; Mei, S.; Zhou, Y. Magnetic Molecularly Imprinted Nanoparticles for Recognition of Lysozyme. Biosens. Bioelectron. 2010, 26, 301–306. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, Y.; Liu, S.; Luo, M.; Du, J.; Fan, J.; Xiong, H.; Peng, H. A Novel Magnetic Fluorescent Molecularly Imprinted Sensor for Highly Selective and Sensitive Detection of 4-Nitrophenol in Food Samples through a Dual-recognition Mechanism. Food Chem. 2021, 348, 129126. [Google Scholar] [CrossRef]
- Da Silva, A.T.M.; Pires, B.C.; Dinali, L.A.F.; Maia, A.C.F.C.; dos Santos, C.J.; Sanches, C.; de Souza Borges, W.; Borges, K.B. Terephthalic Acid-Based Magnetic Molecularly Imprinted Polymer for Enantioselective Capillary Electrophoresis Determination of Atenolol in Human Plasma. Sep. Purif. Technol. 2021, 261, 118257. [Google Scholar] [CrossRef]
- Dinc, M.; Esen, C.; Mizaikoff, B. Recent Advances on Core–Shell Magnetic Molecularly Imprinted Polymers for Biomacromolecules. TrAC Trends Anal. Chem. 2019, 114, 202–217. [Google Scholar] [CrossRef]
- Thomas, J.M. The Chemistry of Crystalline Sponges. Mol. Sieves 1994, 386, 289–290. [Google Scholar] [CrossRef]
- Gu, X.-H.; Xu, R.; Yuan, G.-L.; Lu, H.; Gu, B.R.; Xie, H.-P. Preparation of Chlorogenic Acid Surface-Imprinted Magnetic Nanoparticles and Their Usage in Separation of Traditional Chinese Medicine. Anal. Chim. Acta 2010, 675, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Li, H.; Sun, M.; Fan, L.; Qiu, H.; Li, X.; Luo, C. Flow Injection Chemiluminescence Sensor Based on Core-Shell Magnetic Molecularly Imprinted Nanoparticles for Determination of Sulfadiazine. Anal. Chim. Acta 2012, 718, 84–91. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, G.; Xu, L.; Li, B.; Cai, Q.; Jiang, H.; Zhou, X. Facile and Controllable One-Step Fabrication of Molecularly Imprinted Polymer Membrane by Magnetic Field Directed Self-Assembly for Electrochemical Sensing of Glutathione. Anal. Chim. Acta 2015, 886, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.L.; Gao, S. Solvothermal Reduction Synthesis and Magnetic Properties of Polymer Protected Iron and Nickel Nanocrystals. J. Alloys Compd. 2004, 365, 112–116. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and Characterization of Biocompatible Fe3O4 Nanoparticles. J. Biomed. Mater. Res. Part A 2007, 80, 333–341. [Google Scholar] [CrossRef]
- Jamil, S.; Janjua, M.R.S.A. Synthetic Study and Merits of Fe3O4 Nanoparticles as Emerging Material. J. Clust. Sci. 2017, 28, 2369–2400. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Lin, H.; Abd El-Aty, A.M.; Rabah, T.; Liu, X.; Yu, Z.; Yong, Y.; Ju, X.; She, Y. Magnetic Molecularly Imprinted Specific Solid-Phase Extraction for Determination of Dihydroquercetin from Larix Griffithiana Using HPLC. J. Sep. Sci. 2020, 43, 2301–2310. [Google Scholar] [CrossRef]
- Nakaya, M.; Nishida, R.; Muramatsu, A. Size Control of Magnetite Nanoparticles in Excess Ligands as a Function of Reaction Temperature and Time. Molecules 2014, 19, 11395–11403. [Google Scholar] [CrossRef] [Green Version]
- Maity, D.; Ding, J.; Xue, J.-M. Synthesis of Magnetite Nanoparticles by Thermal Decomposition: Time, Temperature, Surfactant and Solvent Effects. World Sci. Publ. Co. 2008, 1, 189–193. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z.; Hekmatara, H. Green Synthesis of Fe3O4 Nanoparticles and Survey Their Magnetic Properties. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2016, 46, 1047–1052. [Google Scholar] [CrossRef]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large Ph Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dramou, P.; Zuo, P.; He, H.; Pham-Huy, L.A.; Zou, W.; Xiao, D.; Pham-Huy, C. Development of Novel Amphiphilic Magnetic Molecularly Imprinted Polymers Compatible with Biological Fluids for Solid Phase Extraction and Physicochemical Behavior Study. J. Chromatogr. A 2013, 1317, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Dramou, P.; Zuo, P.; He, H.; Pham-Huy, L.A.; Zou, W.; Xiao, D.; Pham-Huy, C.; Ndorbor, T. Anticancer Loading and Controlled Release of Novel Water-Compatible Magnetic Nanomaterials as Drug Delivery Agents, Coupled to a Computational Modeling Approach. J. Mater. Chem. B 2013, 1, 4099–4109. [Google Scholar] [CrossRef]
- Sanadgol, N.; Wackerlig, J. Developments of Smart Drug-Delivery Systems Based on Magnetic Molecularly Imprinted Polymers for Targeted Cancer Therapy: A Short Review. Pharmaceutics 2020, 12, 831. [Google Scholar] [CrossRef]
- Li, D.; Jiang, D.; Chen, M.; Xie, J.; Wu, Y.; Dang, S.; Zhang, J. An Easy Fabrication of Monodisperse Oleic Acid-Coated Fe3O4 Nanoparticles. Mater. Lett. 2010, 64, 2462–2464. [Google Scholar] [CrossRef]
- Tan, Y.; Jin, J.; Zhang, S.; Shi, Z.; Wang, J.; Zhang, J.; Pu, W.; Yang, C. Electrochemical Determination of Bisphenol A Using a Molecularly Imprinted Chitosan-Acetylene Black Composite Film Modified Glassy Carbon Electrode. Electroanalysis 2016, 28, 189–196. [Google Scholar] [CrossRef]
- Karrat, A.; Lamaoui, A.; Amine, A.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. Applications of Chitosan in Molecularly and Ion Imprinted Polymers. Chem. Afr. 2020, 3, 513–533. [Google Scholar] [CrossRef]
- Zheng, X.F.; Lian, Q.; Yang, H.; Wang, X. Surface Molecularly Imprinted Polymer of Chitosan Grafted Poly(Methyl Methacrylate) for 5-Fluorouracil and Controlled Release. Sci. Rep. 2016, 6, 21409. [Google Scholar] [CrossRef] [Green Version]
- Yuwei, C.; Jianlong, W. Preparation and Characterization of Magnetic Chitosan Nanoparticles and Its Application for Cu(II) Removal. Chem. Eng. J. 2011, 168, 286–292. [Google Scholar] [CrossRef]
- Karimi Pasandideh, E.; Kakavandi, B.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Esrafili, A.; Rezaei Kalantary, R. Silica-Coated Magnetite Nanoparticles Core-Shell Spheres (Fe3O4@SiO2) for Natural Organic Matter Removal. J. Environ. Health Sci. Eng. 2016, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dil, E.A.; Doustimotlagh, A.H.; Javadian, H.; Asfaram, A.; Ghaedi, M. Nano-Sized Fe3O4@SiO2-Molecular Imprinted Polymer as a Sorbent for Dispersive Solid-Phase Microextraction of Melatonin in the Methanolic Extract of Portulaca Oleracea, Biological, and Water Samples. Talanta 2021, 221, 121620. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Li, W.; Lei, X.; Fan, X.; Tian, L.; Zhang, H.; Zhang, Q. Preparation and Characterization of Bovine Serum Albumin Surface-Imprinted Thermosensitive Magnetic Polymer Microsphere and Its Application for Protein Recognition. Biosens. Bioelectron. 2014, 51, 261–267. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Liu, R.; Tan, W.; Li, G. Magnetic Molecularly Imprinted Polymer Beads Prepared by Microwave Heating for Selective Enrichment of β-Agonists in Pork and Pig Liver Samples. Talanta 2011, 84, 462–470. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Yuan, D.; Feng, S.; Liu, B. An Automatic Gas-Phase Molecular Absorption Spectrometric System Using a UV-LED Photodiode Based Detector for Determination of Nitrite and Total Nitrate. Talanta 2011, 84, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, N.; Asfaram, A.; Javadian, H.; Haddadi, H.; Sharifpour, E. Ultrasound-Assisted Solid Phase Microextraction-HPLC Method Based on Fe3O4@SiO2-NH2-Molecularly Imprinted Polymer Magnetic Nano-Sorbent for Rapid and Efficient Extraction of Harmaline from Peganum Harmala Extract. J. Chromatogr. B 2021, 1171, 122640. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Drioli, E.; Guzzo, L.; Donato, L. Bio-Mimetic Sensors Based on Molecularly Imprinted Membranes. Sensors 2014, 14, 13863–13912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guć Maria, S.G. The Molecularly Imprinted Polymers. Influence of Monomers on The Properties of Polymers—A Review. World J. Res. Rev. 2017, 5, 36–47. [Google Scholar]
- Safdarian, M.; Ramezani, Z.; Ghadiri, A.A. Facile Synthesis of Magnetic Molecularly Imprinted Polymer: Perphenazine Template and Its Application in Urine and Plasma Analysis. J. Chromatogr. A 2016, 1455, 28–36. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef]
- Anene, A.; Kalfat, R.; Chevalier, Y.; Hbaieb, S. Design of Molecularly Imprinted Polymeric Materials: The Crucial Choice of Functional Monomers. Chem. Afr. 2020, 3, 769–781. [Google Scholar] [CrossRef]
- Suryana, S.; Mutakin, M.; Rosandi, Y.; Hasanah, A.N. Rational Design of Salmeterol Xinafoate Imprinted Polymer through Computational Method: Functional Monomer and Crosslinker Selection. Polym. Adv. Technol. 2021, 33, 221–234. [Google Scholar] [CrossRef]




| Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Co-precipitation |
|
| [97,98] |
| Solvothermal |
|
| [98] |
| Factor | Effect [36,99,100] |
|---|---|
| Reaction temperature | Higher reaction temperature, larger size of nanoparticle [36,99,100], increased magnetisation saturation (MS) [100], more uniform and regular structure [36]. |
| Reaction time | Higher reaction time, larger size of nanoparticle [36,99,100] and increased magnetisation saturation (MS) [100]. |
| Analyte | Modification Component | Q MMIP (μg/g) | Q MNIP (μg/g) | Recovery MMIP (%) | Ref. |
|---|---|---|---|---|---|
| Chloramphenicol | Oleic acid | 5679 | 2922 | 84.3–90.9 | [8] |
| Imidacloprid | Oleic acid | 24,032 | 9.97 | 94.0–98.0 | [82] |
| Metronidazole | Oleic acid | 10,800 | 4920 | 90.6–104.2 in toner sample; 84.1–91.4 in powder sample; and 90.3–100.4 in cream | [49] |
| 6-mercaptopurine (6-MP) and thioguanine (TG) | Oleic acid | 6-MP: 822.29 TG: 519.15 | 6-MP: 537.92 TG: 352.24 | 8.89–103.03 for 6-MP and 85.94–98.27 for TG | [21] |
| Tricyclazole | Chitosan | 45,454.55 | 26,315.79 | 89.4 (rice), 90.9 (water) | [31] |
| Cu(II) | Chitosan | 35,500 | - | - | |
| Resveratrol | Tetraethoxysilane (TEOS) | 5331.92 | - | 79.3–90.6 | [70] |
| Humic acid | Tetraethoxysilane (TEOS) | 196,070 | 96,150 | - | [25] |
| Melatonin | Tetraethoxysilane (TEOS) | 109,100 | 39,040 | 93.07–104.1 | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamruddin, N.M.; Herman, H.; Rijai, L.; Hasanah, A.N. Factors Affecting the Analytical Performance of Magnetic Molecularly Imprinted Polymers. Polymers 2022, 14, 3008. https://doi.org/10.3390/polym14153008
Zamruddin NM, Herman H, Rijai L, Hasanah AN. Factors Affecting the Analytical Performance of Magnetic Molecularly Imprinted Polymers. Polymers. 2022; 14(15):3008. https://doi.org/10.3390/polym14153008
Chicago/Turabian StyleZamruddin, Nur Masyithah, Herman Herman, Laode Rijai, and Aliya Nur Hasanah. 2022. "Factors Affecting the Analytical Performance of Magnetic Molecularly Imprinted Polymers" Polymers 14, no. 15: 3008. https://doi.org/10.3390/polym14153008