Dual-Modified Lignin-Assembled Multilayer Microsphere with Excellent Pb2+ Capture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
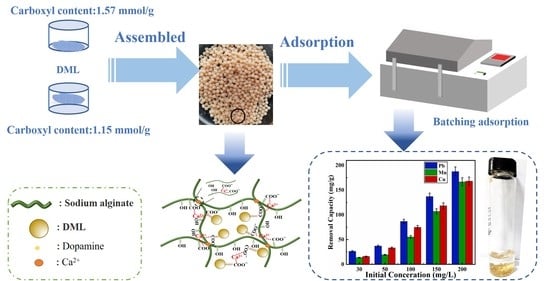
2.2. Methods
2.3. Characterization of the LMM
2.4. Adsorption Experiments
2.5. The Reproducibility of the LMM
3. Results and Discussion
3.1. The Determination of Carboxyl Content in DML
3.2. The Characterization of the LMM
3.3. Adsorption Efficiency of the LMM for Pb2+
3.4. Adsorption Kinetics
3.5. Adsorption Isotherm and Thermodynamics
3.6. Thermodynamics of the LMM Adsorption
3.7. Recyclability of the LMM
3.8. Selectivity of the LMM Adsorption
3.9. Adsorption Mechanism
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.; Sun, H.; Zhu, Y.; Zhang, D.; Wang, L.; Dai, F.; Zhao, Y.; Chen, L. Enhanced selective removal of lead ions using a functionalized PAMAM@UiO-66-NH2 nanocomposite: Experiment and mechanism. Microporous Mesoporous Mater. 2021, 328, 111433–111447. [Google Scholar] [CrossRef]
- Kucherova, A.E.; Romantsova, I.V.; Burakov, A.E.; Memetov, N.R.; Krasnyansky, M.N. Graphene-Based Nanocomposites for Enhanced Pb2+ Adsorption. Nano Hybrids Compos. 2017, 13, 323–329. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shi, X.; Wu, W.; An, X.; Tian, Y.; Qiao, Y. Facile preparation of lignosulfonate/N-methylaniline composite and its application in efficient removal of Cr(VI) from aqueous solutions. Int. J. Biol. Macromol. 2020, 154, 1194–1204. [Google Scholar] [CrossRef]
- Aaa, B.; Shms, B.; Aay, C.; Mnmi, C. Synthesis and characterization of GO-Ag nanocomposite for removal of malachite dye from aqueous solution. Mater. Today Proc. 2021, 47, 1359–1365. [Google Scholar]
- Zhai, C.; Sun, F.; Zhang, P.; Ma, H.; Song, A.; Hao, J. Interactions of dopamine and dopamine hydrochloride with ethanol. J. Mol. Liq. 2016, 223, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.S.; Asif, J.; Liu, L.C.; Wei, S.Y.; Huai-Hsuan, T.; Nitsche, M.A.; Min-Fang, K. Nonlinear Effects of Dopamine D1 Receptor Activation on Visuomotor Coordination Task Performance. Cereb. Cortex 2020, 30, 5346–5355. [Google Scholar] [CrossRef]
- Matt, S.M.; Gaskill, P.J. Where Is Dopamine and how do Immune Cells See it? Dopamine-Mediated Immune Cell Function in Health and Disease. J. Neuroimmune Pharmacol. 2019, 15, 114–164. [Google Scholar] [CrossRef]
- Landgraf, D.; Joiner, W.J.; Mccarthy, M.J.; Kiessling, S.; Barandas, R.; Young, J.W.; Cermakian, N.; Welsh, D.K. The mood stabilizer valproic acid opposes the effects of dopamine on circadian rhythms. Neuropharmacology 2016, 107, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, M.L.B.; Martin, C.S.; Furini, L.N.; Rubira, R.J.G.; Batagin-Neto, A.; Alessio, P.; Constantino, C.J.L. Surface-enhanced Raman scattering for dopamine in Ag colloid: Adsorption mechanism and detection in the presence of interfering species. Appl. Surf. Sci. 2020, 522, 146466–146475. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and Properties of Sodium Carboxymethyl Cellulose/Sodium Alginate/Chitosan Composite Film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Chen, T.; Dong, X.; Mei, Z. Preparation and characterization of composite hydrogel beads based on sodium alginate. Polym. Bull. 2015, 72, 2857–2869. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Z.; Shi, J.; Zhang, C.; Zhang, W.; Wu, H. Dopamine-Modified Alginate Beads Reinforced by Cross-Linking via Titanium Coordination or Self-Polymerization and Its Application in Enzyme Immobilization. Ind. Eng. Chem. Res. 2013, 52, 14828–14836. [Google Scholar] [CrossRef]
- Zhou, X.; Jin, C.; Liu, G.; Wu, G.; Huo, S.; Kong, Z. Functionalized lignin-based magnetic adsorbents with tunable structure for the efficient and selective removal of Pb(II) from aqueous solution. Chem. Eng. J. 2021, 420, 130409–130418. [Google Scholar] [CrossRef]
- Ku, A.; Aay, A.; Mnmi, A.; Tpb, C.; Tus, A. Environmental applications of smart polymer composites. Smart Polym. Nanocompos. 2021, 15, 295–312. [Google Scholar]
- Popovic, A.L.; Rusmirovic, J.D.; Velickovic, Z.; Kovacevic, T.; Jovanovic, A.; Cvijetic, I.; Marinkovic, A.D. Kinetics and column adsorption study of diclofenac and heavy-metal ions removal by amino-functionalized lignin microspheres. J. Ind. Eng. Chem. 2021, 93, 302–314. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; Jie, P.; Zhu, J.; Cheng, F. Preparation of chitosan/lignosulfonate for effectively removing Pb(II) in water. Polymer 2021, 228, 123878–123888. [Google Scholar] [CrossRef]
- Pang, Y.; Chen, Z.; Zhao, R.; Yi, C.; Qiu, X.; Qian, Y.; Lou, H. Facile synthesis of easily separated and reusable silver nanoparticles/aminated alkaline lignin composite and its catalytic ability. J. Colloid Interface Sci. 2021, 587, 334–346. [Google Scholar] [CrossRef]
- Ge, Y.; Qin, L.; Li, Z. Lignin microspheres: An effective and recyclable natural polymer-based adsorbent for lead ion removal. Mater. Des. 2016, 95, 141–147. [Google Scholar] [CrossRef]
- Wu, F.; Chen, L.; Hu, P.; Wang, Y.; Deng, J.; Mi, B. Industrial alkali lignin-derived biochar as highly efficient and low-cost adsorption material for Pb(II) from aquatic environment. Bioresour. Technol. 2021, 322, 124539–124547. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Klapiszewski, L.; Moszynski, D.; Bartczak, P.; Szatkowski, T.; Majchrzak, I.; Siwinska-Stefanska, K.; Bazhenov, V.V.; Jesionowski, T. Modification of chitin with kraft lignin and development of new biosorbents for removal of cadmium(II) and nickel(II) ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Wu, T.; Fang, G.; Ran, M.; Shen, K.; Liao, G. Self-assembly preparation of lignin–graphene oxide composite nanospheres for highly efficient Cr(vi) removal. RSC Adv. 2021, 11, 4713–4722. [Google Scholar] [CrossRef]
- Popovic, A.L.; Rusmirovic, J.D.; Velickovic, Z.; Radovanovic, Z.; Ristic, M.; Pavlovic, V.P.; Marinkovic, A.D. Novel amino-functionalized lignin microspheres: High performance biosorbent with enhanced capacity for heavy metal ion removal. Int. J. Biol. Macromol. 2020, 156, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, S.; Wang, X.; Zhang, W.; Lagerquist, L.; Qin, M.; Willför, S.; Xu, C.; Fatehi, P. Ultrafast adsorption of heavy metal ions onto functionalized lignin-based hybrid magnetic nanoparticles. Chem. Eng. J. 2019, 372, 82–91. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, A.; Li, D.; Shi, L.; Luo, Z.; Peng, C. Simultaneous removal of methylene blue and Pb(2+) from aqueous solution by adsorption on facile modified lignosulfonate. Environ. Technol. 2020, 41, 1677–1690. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Zhong, X.; Zhuo, Z.; Tian, S.; Fu, S.; Chen, Y.; Liu, Y. Constructing MoS2/Lignin-derived carbon nanocomposites for highly efficient removal of Cr(VI) from aqueous environment. J. Hazard. Mater. 2021, 408, 124847–124857. [Google Scholar] [CrossRef]
- Stanisz, M.; Klapiszewski, L.; Kolodynska, D.; Jesionowski, T. Development of functional lignin-based spherical particles for the removal of vanadium(V) from an aqueous system. Int. J. Biol. Macromol. 2021, 186, 181–193. [Google Scholar] [CrossRef]
- Meng, Y.; Li, C.; Liu, X.; Lu, J.; Cheng, Y.; Xiao, L.P.; Wang, H. Preparation of magnetic hydrogel microspheres of lignin derivate for application in water. Sci. Total Environ. 2019, 685, 847–855. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, C.; Ding, Q.; Yu, D.; Li, R. Novel dual modified alkali lignin based adsorbent for the removal of Pb2+ in water. Ind. Crops Prod. 2021, 173, 114100–114110. [Google Scholar] [CrossRef]
- Tao, E.; Ma, D.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Compd. 2020, 832, 154833. [Google Scholar]
- Shi, X.; Hong, J.; Li, J.; Kong, S.; Song, G.; Naik, N.; Guo, Z. Excellent selectivity and high capacity of As (V) removal by a novel lignin-based adsorbent doped with N element and modified with Ca(2+). Int. J. Biol. Macromol. 2021, 172, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Hou, Y. Preparation of a Novel Lignin Nanosphere Adsorbent for Enhancing Adsorption of Lead. Molecules 2019, 24, 2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, C.; Zhang, X.; Xin, J.; Liu, G.; Wu, G.; Kong, Z.; Zhang, J. Clickable Synthesis of 1,2,4-Triazole Modified Lignin-Based Adsorbent for the Selective Removal of Cd(II). Sustain. Chem. Eng. 2017, 5, 4086–4093. [Google Scholar] [CrossRef]
- Sohni, S.; Hashim, R.; Nidaullah, H.; Lamaming, J.; Sulaiman, O. Chitosan/nano-lignin based composite as a new sorbent for enhanced removal of dye pollution from aqueous solutions. Int. J. Biol. Macromol. 2019, 132, 1304–1317. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Peng, L.; Han, S.; Hao, C.; Jiang, C.; Wang, H.; Fan, X. Effective removal of heavy metals from water using porous lignin-based adsorbents. Chemosphere 2021, 279, 130504–130514. [Google Scholar] [CrossRef]
- Qin, L.; Ge, Y.; Deng, B.; Li, Z. Poly (ethylene imine) anchored lignin composite for heavy metals capturing in water. J. Taiwan Inst. Chem. Eng. 2017, 71, 84–90. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Issaoui, H.; Sallem, F.; Lafaille, J.; Grassl, B.; Charrier–El Bouhtoury, F. Biosorption of Heavy Metals from Water onto Phenolic Foams Based on Tannins and Lignin Alkaline Liquor. Int. J. Environ. Res. 2021, 15, 369–381. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, C.; Zhang, J.; He, F.; Yao, Y.; Zhang, T.C.; He, C. Insights into the adsorption of Pb(II) over trimercapto-s-triazine trisodium salt-modified lignin in a wide pH range. Chem. Eng. J. Adv. 2020, 1, 100002–100013. [Google Scholar] [CrossRef]
- Jin, C.; Liu, G.; Wu, G.; Huo, S.; Liu, Z.; Kong, Z. Facile fabrication of crown ether functionalized lignin-based biosorbent for the selective removal of Pb(II). Ind. Crops Prod. 2020, 155, 112829–112837. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Hou, Y. Preparation of magnetic polyethylenimine lignin and its adsorption of Pb(II). Int. J. Biol. Macromol. 2019, 141, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; He, S.; Hou, H.; Hao, C. Ultrasonic-assisted synthesis of superabsorbent hydrogels based on sodium lignosulfonate and their adsorption properties for Ni(2+). Ultrason. Sonochem. 2018, 40, 221–229. [Google Scholar] [CrossRef]
- Ge, Y.; Xiao, D.; Li, Z.; Cui, X. Dithiocarbamate functionalized lignin for efficient removal of metallic ions and the usage of the metal-loaded bio-sorbents as potential free radical scavengers. J. Mater. Chem. A 2014, 2, 2136–2145. [Google Scholar] [CrossRef]
- Bo, S.; Luo, J.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Efficiently selective adsorption of Pb(Ⅱ) with functionalized alginate-based adsorbent in batch/column systems: Mechanism and application simulation. J. Clean. Prod. 2020, 250, 119585–119603. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Zeng, H.; Rhimi, B.; Wang, C. Novel polyethyleneimine functionalized chitosan–lignin composite sponge with nanowall-network structures for fast and efficient removal of Hg(ii) ions from aqueous solution. Environ. Sci. Nano 2020, 7, 793–802. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, C.; Cui, W.; He, F.; Zhang, J.; Zhang, T.C.; He, C. Adsorption of Pb2+ and Cu2+ ions on the CS2-modified alkaline lignin. Chem. Eng. J. 2020, 391, 123581–123592. [Google Scholar] [CrossRef]









| Model | Pseudo-First-Order | ||
|---|---|---|---|
| C0 (mg/L) | qe (mg/g) | k1 (min−1) | R2 |
| 30 | 22.08 | 0.02349 | 0.9696 |
| Pseudo-second-order | |||
| C0 (mg/L) | qe (mg/g) | k2 (g/mg min) | R2 |
| 30 | 38.91 | 0.001929 | 0.9999 |
| intraparticle diffusion model | |||
| C | Kp | R2 | |
| Step 1 | 10.29 | 2.739 | 0.9053 |
| Step 2 | 20.65 | 1.252 | 0.9274 |
| Step 3 | 36.04 | 0.05840 | 0.8944 |
| Model | Langmuir Model | ||
|---|---|---|---|
| T (K) | qmax (mg/g) | KL (L/mg) | R2 |
| 303 | 185.19 | 0.07649 | 0.7248 |
| 313 | 204.81 | 0.1012 | 0.7721 |
| 323 | 250.0 | 0.2353 | 0.9422 |
| Freundlich model | |||
| T (K) | KF (L/g) | 1/n | R2 |
| 303 | 40.85 | 0.3011 | 0.8130 |
| 313 | 46.53 | 0.3266 | 0.8783 |
| 323 | 58.56 | 0.4088 | 0.9900 |
| Adsorbent | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| chitosan/lignosulfonate hydrogel | 525 | [18] |
| Bio-sourced phenolic foams | 100.9 | [39] |
| Cross-linked lignosulfonate bio-adsorbent | 64.9 | [26] |
| Modified alkaline lignin | 73.7 | [40] |
| Functionalized lignin-based adsorbent | 91.4 | [41] |
| Lignin-based hybrid magnetic nanoparticles | 150.33 | [25] |
| Magnetic polyethyleneimine lignin | 96.60 | [42] |
| LMM | 250.0 | This work |
| T (K) | ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (KJ/mol/K) |
|---|---|---|---|
| 303 | −28.89 | 16.26 | 0.149 |
| 313 | −30.38 | ||
| 323 | −31.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chen, Y.; Wu, C. Dual-Modified Lignin-Assembled Multilayer Microsphere with Excellent Pb2+ Capture. Polymers 2022, 14, 2824. https://doi.org/10.3390/polym14142824
Zhang Z, Chen Y, Wu C. Dual-Modified Lignin-Assembled Multilayer Microsphere with Excellent Pb2+ Capture. Polymers. 2022; 14(14):2824. https://doi.org/10.3390/polym14142824
Chicago/Turabian StyleZhang, Zhaohui, Yehong Chen, and Chaojun Wu. 2022. "Dual-Modified Lignin-Assembled Multilayer Microsphere with Excellent Pb2+ Capture" Polymers 14, no. 14: 2824. https://doi.org/10.3390/polym14142824