Sol-Gel Assisted Immobilization of Alizarin Red S on Polyester Fabrics for Developing Stimuli-Responsive Wearable Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Preparation of Halochromic Fabrics and Method of Testing
2.2. Chemical–Physical Characterizations
3. Results and Discussion
3.1. ATR-FTIR Characterization and Washing Fastness Evaluation of Dyed Textile Fabrics
3.2. Surface Chemical Composition of Textile Fabrics by XPS
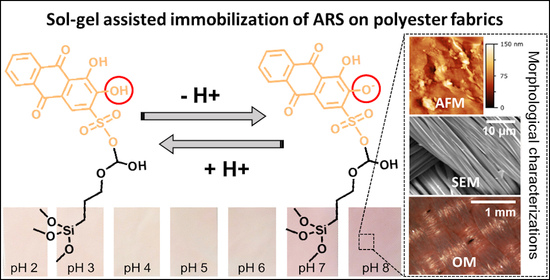
3.3. Morphological Characterization of Untreated and Treated Textiles
3.4. Diffuse Reflectance Measurements: Textile pH Response
3.5. Dynamic Response of Halochromic Coatings to Changes in pH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruckdashel, R.R.; Venkataraman, D.; Park, J.H. Smart textiles: A toolkit to fashion the future. J. Appl. Phys. 2021, 129, 130903. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abdelrahman, M.S. From Smart Materials to Chromic Textiles. In Advances in Functional Finishing of Textiles; Springer: Berlin/Heidelberg, Germany, 2020; pp. 257–274. [Google Scholar]
- Alaysuy, O.; Snari, R.M.; Alfi, A.A.; Aldawsari, A.M.; Abu-Melha, S.; Khalifa, M.E.; El-Metwaly, N.M. Development of green and sustainable smart biochromic and therapeutic bandage using red cabbage (Brassica oleracea L. Var. capitata) extract encapsulated into alginate nanoparticles. Int. J. Biol. Macromol. 2022, 211, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Abou-Yousef, H.; Khattab, T.A.; Youssef, Y.A.; Al-Balakocy, N.; Kamel, S. Novel cellulose-based halochromic test strips for naked-eye detection of alkaline vapors and analytes. Talanta 2017, 170, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, S.D.; Snari, R.M.; Al-Ahmed, Z.A.; Hossan, A.; Munshi, A.M.; Alfi, A.A.; El-Metwaly, N.M. Novel halochromic hydrazonal chromophore immobilized into rice-straw based cellulose aerogel for vapochromic detection of ammonia. J. Mol. Liq. 2022, 350, 118539. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Fouda, M.M.G.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Khattab, T.A. Development of colorimetric cotton swab using molecular switching hydrazone probe in calcium alginate. J. Mol. Struct. 2020, 1216, 128301. [Google Scholar] [CrossRef]
- Trovato, V.; Colleoni, C.; Castellano, A.; Plutino, M.R. The key role of 3-glycidoxypropyltrimethoxysilane sol–gel precursor in the development of wearable sensors for health monitoring. J. Sol-Gel Sci. Technol. 2018, 87, 27–40. [Google Scholar] [CrossRef]
- Trovato, V.; Vitale, A.; Bongiovanni, R.; Ferri, A.; Rosace, G.; Plutino, M.R. Development of a Nitrazine Yellow-glycidyl methacrylate coating onto cotton fabric through thermal-induced radical polymerization reactions: A simple approach towards wearable pH sensors applications. Cellulose 2021, 28, 3847–3868. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef] [PubMed]
- Getu, A.; Sahu, O. Technical Fabric as Health Care Material. Biomed. Sci. Eng. 2014, 2, 35–39. [Google Scholar]
- Nimbekar, A.A.; Deshmukh, R.R. Plasma-induced grafting of polyaniline on polyester fabric for gas sensing application. J. Mater. Sci. Mater. Electron. 2021, 32, 59–72. [Google Scholar] [CrossRef]
- Rahman, M. Mashiur Degradation of Polyesters in Medical Applications. In Polyester; InTech: London, UK, 2012. [Google Scholar]
- Boukhriss, A.; El Messoudi, M.; Roblin, J.-P.; Aaboub, T.; Boyer, D.; Gmouh, S. Luminescent hybrid coatings prepared by a sol–gel process for a textile-based pH sensor. Mater. Adv. 2020, 1, 918–925. [Google Scholar] [CrossRef]
- Vatansever, F.; Burtovyy, R.; Zdyrko, B.; Ramaratnam, K.; Andrukh, T.; Minko, S.; Owens, J.R.; Kornev, K.G.; Luzinov, I. Toward Fabric-Based Flexible Microfluidic Devices: Pointed Surface Modification for pH Sensitive Liquid Transport. ACS Appl. Mater. Interfaces 2012, 4, 4541–4548. [Google Scholar] [CrossRef] [PubMed]
- Salem, T.; Simon, F.; El-Sayed, A.A.; Salama, M. Plasma-assisted surface modification of polyester fabric for developing halochromic properties. Fibers Polym. 2017, 18, 731–740. [Google Scholar] [CrossRef]
- Van der Schueren, L.; De Clerck, K. Textile materials with a pH-sensitive function. Int. J. Cloth. Sci. Technol. 2011, 23, 269–274. [Google Scholar] [CrossRef]
- Guido, E.; Alongi, J.; Colleoni, C.; Di Blasio, A.; Carosio, F.; Verelst, M.; Malucelli, G.; Rosace, G. Thermal stability and flame retardancy of polyester fabrics sol–gel treated in the presence of boehmite nanoparticles. Polym. Degrad. Stab. 2013, 98, 1609–1616. [Google Scholar] [CrossRef]
- Irena, G.; Jolanta, B.; Karolina, Z. Chemical modification of poly(ethylene terephthalate) and immobilization of the selected enzymes on the modified film. Appl. Surf. Sci. 2009, 255, 8293–8298. [Google Scholar] [CrossRef]
- Kan, C.W.; Yuen, C.W.M. Effect of atmospheric pressure plasma treatment on wettability and dryability of synthetic textile fibres. Surf. Coat. Technol. 2013, 228, S607–S610. [Google Scholar] [CrossRef]
- Guido, E.; Colleoni, C.; De Clerck, K.; Plutino, M.R.; Rosace, G. Influence of catalyst in the synthesis of a cellulose-based sensor: Kinetic study of 3-glycidoxypropyltrimethoxysilane epoxy ring opening by Lewis acid. Sens. Actuators B Chem. 2014, 203, 213–222. [Google Scholar] [CrossRef]
- Trovato, V.; Mezzi, A.; Brucale, M.; Rosace, G.; Plutino, M.R. Alizarin-functionalized organic-inorganic silane coatings for the development of wearable textile sensors. J. Colloid Interface Sci. 2022, 617, 463–477. [Google Scholar] [CrossRef]
- Plutino, M.R.; Colleoni, C.; Donelli, I.; Freddi, G.; Guido, E.; Maschi, O.; Mezzi, A.; Rosace, G. Sol-gel 3-glycidoxypropyltriethoxysilane finishing on different fabrics: The role of precursor concentration and catalyst on the textile performances and cytotoxic activity. J. Colloid Interface Sci. 2017, 506, 504–517. [Google Scholar] [CrossRef]
- Ekanayake, U.G.M.; Dissanayake, D.M.S.N.; Rathuwadu, N.; Kumarasinghe, R.K.K.G.R.; Rodrigo, S.K.; Mantilaka, M.M.M.G.P.G. Facile fabrication of fluoro-polymer self-assembled ZnO nanoparticles mediated, durable and robust omniphobic surfaces on polyester fabrics. J. Fluor. Chem. 2020, 235, 109565. [Google Scholar] [CrossRef]
- Küçük, M.; Öveçoğlu, M.L. Surface modification and characterization of polyester fabric by coating with low temperature synthesized ZnO nanorods. J. Sol-Gel Sci. Technol. 2018, 88, 345–358. [Google Scholar] [CrossRef]
- Ingo, G.M.; Kaciulis, S.; Mezzi, A.; Valente, T.; Gusmano, G. Surface characterization of titanium nitride composite coatings fabricated by reactive plasma spraying. Surf. Interface Anal. 2004, 36, 1147–1150. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- CIELAB. Color Space, Application Notes, Hunter Lab 8 (7). 1996. Available online: http://lib3.dss.go.th/fulltext/glass/GlassTheories/CIE_Lab_info.pdf (accessed on 21 January 2022).
- Devarayan, K.; Kim, B.-S. Reversible and universal pH sensing cellulose nanofibers for health monitor. Sens. Actuators B Chem. 2015, 209, 281–286. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, A.; Xie, K.; Gao, A. Smart color-changing paper packaging sensors with pH sensitive chromophores based on azo-anthraquinone reactive dyes. Sens. Actuators B Chem. 2019, 286, 362–369. [Google Scholar] [CrossRef]
- Allahyarzadeh, V.; Montazer, M.; Nejad, N.H.; Samadi, N. In situ synthesis of nano silver on polyester using NaOH/Nano TiO2. J. Appl. Polym. Sci. 2013, 129, 892–900. [Google Scholar] [CrossRef]
- Kale, K.H.; Palaskar, S.S. Plasma enhanced chemical vapor deposition of tetraethylorthosilicate and hexamethyldisiloxane on polyester fabrics under pulsed and continuous wave discharge. J. Appl. Polym. Sci. 2012, 125, 3996–4006. [Google Scholar] [CrossRef]
- Ketema, A.; Worku, A. Review on Intermolecular Forces between Dyes Used for Polyester Dyeing and Polyester Fiber. J. Chem. 2020, 2020, 6628404. [Google Scholar] [CrossRef]
- Sharma, R.; Kamal, A.; Mahajan, R.K. A quantitative appraisal of the binding interactions between an anionic dye, Alizarin Red S, and alkyloxypyridinium surfactants: A detailed micellization, spectroscopic and electrochemical study. Soft Matter 2016, 12, 1736–1749. [Google Scholar] [CrossRef]
- Lemlikchi, W.; Sharrock, P.; Fiallo, M.; Nzihou, A.; Mecherri, M.-O. Hydroxyapatite and Alizarin Sulfonate ARS Modeling Interactions for Textile Dyes Removal from Wastewaters. Procedia Eng. 2014, 83, 378–385. [Google Scholar] [CrossRef]
- Shang, S.-M.; Li, Z.; Xing, Y.; Xin, J.H.; Tao, X.-M. Preparation of durable hydrophobic cellulose fabric from water glass and mixed organosilanes. Appl. Surf. Sci. 2010, 257, 1495–1499. [Google Scholar] [CrossRef]
- Plutino, M.R.; Guido, E.; Colleoni, C.; Rosace, G. Effect of GPTMS functionalization on the improvement of the pH-sensitive methyl red photostability. Sens. Actuators B Chem. 2017, 238, 281–291. [Google Scholar] [CrossRef]







| PL_ARSZ | PL_GPTMS-ARS | |||
|---|---|---|---|---|
| Add-on (%) |  |  | ||
| 1W | 5W | 1W | 5W | |
| WLW (%) |  |  |  |  |
| Fdye (%) | ||||
| Sample Code | |||||||
|---|---|---|---|---|---|---|---|
| Chemical Composition (at %) | PL_UT | PL_ARS | PL_GPTMS-ARS | ||||
| UW | 1W | 5W | UW | 1W | 5W | ||
| C–C | 52.0 | 45.5 | 42.6 | - | 43.9 | 41.4 | 41.9 |
| C–O, C=O | 13.1 | 13.8 | 14.3 | - | 15.3 | 16.7 | 16.4 |
| COOR | 10.9 | 11.3 | 10.8 | - | 9.6 | 10.7 | 10.8 |
| SiO2, O–Si–C | - | - | - | - | 2.8 | 2.9 | 2.8 |
| Si/C | - | - | - | - | 0.04 | 0.04 | 0.04 |
| Sample | Unwashed Sample | After 1 Washing Cycle | After 5 Washing Cycles |
|---|---|---|---|
| PL_ARS | 1.07 | - | - |
| PL_GPTMS-ARS | 1.08 | 0.06 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trovato, V.; Mezzi, A.; Brucale, M.; Abdeh, H.; Drommi, D.; Rosace, G.; Plutino, M.R. Sol-Gel Assisted Immobilization of Alizarin Red S on Polyester Fabrics for Developing Stimuli-Responsive Wearable Sensors. Polymers 2022, 14, 2788. https://doi.org/10.3390/polym14142788
Trovato V, Mezzi A, Brucale M, Abdeh H, Drommi D, Rosace G, Plutino MR. Sol-Gel Assisted Immobilization of Alizarin Red S on Polyester Fabrics for Developing Stimuli-Responsive Wearable Sensors. Polymers. 2022; 14(14):2788. https://doi.org/10.3390/polym14142788
Chicago/Turabian StyleTrovato, Valentina, Alessio Mezzi, Marco Brucale, Hamed Abdeh, Dario Drommi, Giuseppe Rosace, and Maria Rosaria Plutino. 2022. "Sol-Gel Assisted Immobilization of Alizarin Red S on Polyester Fabrics for Developing Stimuli-Responsive Wearable Sensors" Polymers 14, no. 14: 2788. https://doi.org/10.3390/polym14142788