Study of the Preparation and Properties of Chrysin Binary Functional Monomer Molecularly Imprinted Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
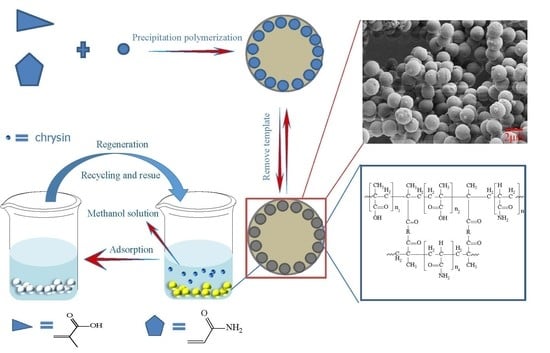
2.2. Preparation of Bi-MIPs, Bi-NIPs, Si-MIPs and Si-NIPs
2.3. Characterization of Bi-MIPs, Bi-NIPs, Si-MIPs and Si-NIPs
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Diameters of the Bi-MIPs, Bi-NIPs, Si-MIPs and Si-NIPs
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Nitrogen Adsorption/Desorption Analysis (BET)
2.4. Static Adsorption
2.4.1. Adsorption Isotherm of Chrysin on Bi-MIPs, Bi-NIPs, Si-MIPs and Si-NIPs
2.4.2. Adsorption Kinetics
2.4.3. Adsorption Thermodynamics
2.5. Selective Adsorption
2.6. Adsorption Reusability
3. Results and Discussion
3.1. Characterization of Bi-MIPs, Bi-NIPs, Si-MIPs and Si-NIPs
3.1.1. SEM
3.1.2. FT-IR
3.1.3. TGA
3.2. Static Adsorption Experiments
3.2.1. Adsorption Isotherm
3.2.2. Adsorption Kinetics
3.2.3. Adsorption Thermodynamics
3.3. Adsorption Selectivity
3.4. Adsorption Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ong, S.; Shanmugam, M.; Fan, L.; Fraser, S.; Arfuso, F.; Ahn, K.; Sethi, G.; Bishayee, A. Focus on Formononetin: Anticancer Potential and Molecular Targets. Cancers 2019, 11, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Wu, F.; Lin, X.; Shen, L.; Feng, Y. Developments in drug delivery of bioactive alkaloids derived from traditional Chinese medicine. Drug Deliv. 2018, 25, 398–416. [Google Scholar] [CrossRef] [Green Version]
- Subramanya, S.B.; Venkataraman, B.; Meeran, M.F.N.; Goyal, S.N.; Patil, C.R.; Ojha, S. Therapeutic Potential of Plants and Plant Derived Phytochemicals against Acetaminophen-Induced Liver Injury. Int. J. Mol. Sci. 2018, 19, 3776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, P.; Rao, V.S.; Hong, Y.-A.; Reddy, P.M. Molecular Design, Synthesis, and Biological Evaluation of 2-Hydroxy-3-Chrysino Dithiocarbamate Derivatives. Molecules 2019, 24, 3038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Oudat, B.; Ramapuram, H.; Malla, S.; Audat, S.; Hussein, N.; Len, J.; Kumari, S.; Bedi, M.; Ashby, C.; Tiwari, A. Novel Chrysin-De-Allyl PAC-1 Hybrid Analogues as Anticancer Compounds: Design, Synthesis, and Biological Evaluation. Molecules 2020, 25, 3063. [Google Scholar] [CrossRef]
- Liu, C.; Kou, X.; Wang, X. Novel chrysin derivatives as hidden multifunctional agents for anti-Alzheimer’s disease: Design, synthesis and in vitro evaluation. Eur. J. Pharm. Sci. 2021, 166, 105976. [Google Scholar] [CrossRef]
- Yu, C.-H.; Suh, B.; Shin, I.; Kim, E.-H.; Kim, D.; Shin, Y.-J.; Chang, S.-Y.; Baek, S.-H.; Kim, H.; Bae, O.-N. Inhibitory Effects of a Novel Chrysin-Derivative, CPD 6, on Acute and Chronic Skin Inflammation. Int. J. Mol. Sci. 2019, 20, 2607. [Google Scholar] [CrossRef] [Green Version]
- Maruhashi, R.; Eguchi, H.; Akizuki, R.; Hamada, S.; Furuta, T.; Matsunaga, T.; Endo, S.; Ichihara, K.; Ikari, A. Chrysin enhances anticancer drug-induced toxicity mediated by the reduction of claudin-1 and 11 expression in a spheroid culture model of lung squamous cell carcinoma cells. Sci. Rep. 2019, 9, 13753. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Shi, H.; Liu, D. Chrysin protects against renal ischemia reperfusion induced tubular cell apoptosis and inflammation in mice. Exp. Ther. Med. 2019, 17, 2256–2262. [Google Scholar] [CrossRef] [Green Version]
- Schindler, R.M.R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J. Nutr. 2006, 136, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- Gilles Comte, J.B.D.; Bayet, C. C-Isoprenylation of Flavonoids Enhances Binding Affinity toward P-Glycoprotein and Modulation of Cancer Cell Chemoresistance. J. Med. Chem. 2001, 44, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Gharari, Z.; Bagheri, K.; Danafar, H.; Sharafi, A. Simultaneous determination of baicalein, chrysin and wogonin in four Iranian Scutellaria species by high performance liquid chromatography. J. Appl. Res. Med. Aromat. Plants 2019, 16, 100232. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Wang, R. Comparative two-dimensional HepG2 and L02/cell membrane chromatography/C18/time-of-flight mass spectrometry for screening selective anti-hepatoma components from Scutellariae Radix. J. Pharm. Biomed. Anal. 2019, 164, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Reza, H.; Saman, N.; Kamyar, K. Determination of Flavonoid Markers in Honey with SPE and LC using Experimental Design. Chromatographia 2009, 69, 1291–1297. [Google Scholar]
- Maciejewicz, W.; Daniewski, M.; Bal, K.; Markowski, W. GC-MS identification of the flavonoid aglycones isolated from propolis. Chromatographia 2001, 53, 343–346. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Jia, Z.; Scarlett, C.J.; Sheng, Z. Adsorption/desorption characteristics and enrichment of quercetin, luteolin and apigenin from Flos populi using macroporous resin. Rev. Bras. Farm. 2018, 29, 69–76. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Guo, M. Stainless Steel Wire Mesh Supported Molecularly Imprinted Composite Membranes for Selective Separation of Ebracteolata Compound B from Euphorbia fischeriana. Molecules 2019, 24, 565. [Google Scholar] [CrossRef] [Green Version]
- Kamaruzaman, S.; Nasir, N.M.; Faudzi, S.M.M.; Yahaya, N.; Hanapi, N.S.M.; Ibrahim, W.N.W. Solid-Phase Extraction of Active Compounds from Natural Products by Molecularly Imprinted Polymers: Synthesis and Extraction Parameters. Polymers 2021, 13, 3780. [Google Scholar] [CrossRef]
- Ariani, M.D.; Zuhrotun, A.; Manesiotis, P.; Hasanah, A.N. Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products. Polymers 2022, 14, 1389. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Zhou, Y.; Lian, X.; Yan, L.; Pan, T.; Jin, T.; Xie, H.; Liang, Z.; Qiu, W.; et al. NPCDR: Natural product-based drug combination and its disease-specific molecular regulation. Nucleic Acids Res. 2021, 50, D1324–D1333. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Guo, Q.; Li, P.; Yang, H. A hydrophobic deep eutectic solvents-based integrated method for efficient and green extraction and recovery of natural products from Rosmarinus officinalis leaves, Ginkgo biloba leaves and Salvia miltiorrhiza roots. Food Chem. 2021, 363, 130282. [Google Scholar] [CrossRef] [PubMed]
- Luliński, P.; Maciejewska, D. Examination of Imprinting Process with Molsidomine as a Template. Molecules 2009, 14, 2212–2225. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.M.; Van Grinsven, B.; Foster, C.W.; Cleij, T.J.; Banks, C.E. Introducing Thermal Wave Transport Analysis (TWTA): A Thermal Technique for Dopamine Detection by Screen-Printed Electrodes Functionalized with Molecularly Imprinted Polymer (MIP) Particles. Molecules 2016, 21, 552. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Cong, J.; Wei, B.; Wang, S. Mechanism Analysis of Selective Adsorption and Specific Recognition by Molecularly Imprinted Polymers of Ginsenoside Re. Polymers 2018, 10, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Bai, L.-Y.; Liu, K.-F.; Liu, R.-Q.; Zhang, Y.-P. Atrazine Molecular Imprinted Polymers: Comparative Analysis by Far-Infrared and Ultraviolet Induced Polymerization. Int. J. Mol. Sci. 2014, 15, 574–587. [Google Scholar] [CrossRef]
- Si, Z.; Yu, P.; Dong, Y.; Lu, Y.; Tan, Z.; Yu, X.; Zhao, R.; Yan, Y. Thermo-Responsive Molecularly Imprinted Hydrogels for Selective Adsorption and Controlled Release of Phenol From Aqueous Solution. Front. Chem. 2019, 6, 674. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.; Ma, Y.; Wang, Y.; Wen, Y.; Liu, Z. Specific recognition of proteins and peptides via controllable oriented surface imprinting of boronate affinity-anchored epitopes. Chem. Sci. 2018, 10, 1831–1835. [Google Scholar] [CrossRef] [Green Version]
- Piletska, E.; Yawer, H.; Canfarotta, F.; Moczko, E.; Smolinska-Kempisty, K.; Piletsky, S.S.; Guerreiro, A.; Whitcombe, M.J.; Piletsky, S.A. Biomimetic Silica Nanoparticles Prepared by a Combination of Solid-Phase Imprinting and Ostwald Ripening. Sci. Rep. 2017, 7, 11537. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Poma, A. Advances in Molecularly Imprinted Polymers as Drug Delivery Systems. Molecules 2021, 26, 3589. [Google Scholar] [CrossRef]
- Soufi, G.J.; Iravani, S.; Varma, R.S. Molecularly imprinted polymers for the detection of viruses: Challenges and opportunities. Analyst 2021, 146, 3087–3100. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, W.-C.; Li, Q.; Khan, M.R.; Hu, G.-H.; Liu, Y.; Wu, W.; Huang, C.-X.; Li, R.K. Recent advances in superhydrophobic polyurethane: Preparations and applications. Adv. Colloid Interface Sci. 2022, 303, 102644. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Y.; Li, H. Specific recognition of protein by deep eutectic solvent-based magnetic beta-cyclodextrin molecularly imprinted polymer. Mikrochim. Acta 2021, 188, 232. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xiao, N.; Li, L.; Xie, X.; Li, Y. Theoretical Insight into the Interaction between Chloramphenicol and Functional Monomer (Methacrylic Acid) in Molecularly Imprinted Polymers. Int. J. Mol. Sci. 2020, 21, 4139. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, J.; Wang, J.; Chen, L. Quercetin molecularly imprinted polymers: Preparation, recognition characteristics and properties as sorbent for solid-phase extraction. Talanta 2009, 80, 694–702. [Google Scholar] [CrossRef]
- Sobiech, M.; Luliński, P.; Wieczorek, P.P. Quantum and carbon dots conjugated molecularly imprinted polymers as advanced nanomaterials for selective recognition of analytes in environmental, food and biomedical applications. TrAC Trends Anal. Chem. 2021, 142, 116306. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, M.; Fu, Q.; Wang, L.; Wang, D.; Zhang, K.; Xia, Z.; Gao, D. Novel dual functional monomers based molecularly imprinted polymers for selective extraction of myricetin from herbal medicines. J. Chromatogr. B 2018, 1097–1098, 1–9. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, Y.; Liu, X.; Kong, J.; Nie, C. Preparation of molecular imprinted polymers using bi-functional monomer and bi-crosslinker for solid-phase extraction of rutin. Talanta 2012, 93, 172–181. [Google Scholar] [CrossRef]
- Thach, U.D.; Thi, H.H.N.; Pham, T.D.; Mai, H.D.; Nhu-Trang, T.-T. Synergetic Effect of Dual Functional Monomers in Molecularly Imprinted Polymer Preparation for Selective Solid Phase Extraction of Ciprofloxacin. Polymers 2021, 13, 2788. [Google Scholar] [CrossRef]
- Gao, W.-C.; Wu, W.; Chen, C.-Z.; Zhao, H.; Liu, Y.; Li, Q.; Huang, C.-X.; Hu, G.-H.; Wang, S.-F.; Shi, D.; et al. Design of a Superhydrophobic Strain Sensor with a Multilayer Structure for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2021, 14, 1874–1884. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Zhou, L.; Jiang, S.; Liu, X.; Zhao, H.; Huang, Q.; Wang, L.; Chen, G.; Wang, S. Highly efficient and selective modification of lignin towards optically designable and multifunctional lignocellulose nanopaper for green light-management applications. Int. J. Biol. Macromol. 2022, 206, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Li, L. Preparation of Rosin-Based Composite Membranes and Study of Their Dencichine Adsorption Properties. Polymers 2022, 14, 2161. [Google Scholar] [CrossRef] [PubMed]
- Zahara, S.; Minhas, M.A.; Shaikh, H.; Ali, M.S.; Bhanger, M.I.; Malik, M.I. Molecular imprinting-based extraction of rosmarinic acid from Salvia hypoleuca extract. React. Funct. Polym. 2021, 166, 104984. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Liu, X.; Yang, Q.; Huang, Q.; Wang, L.; Dai, Y.; Qin, C.; Wang, S. Highly Transparent, UV-Shielding, and Water-Resistant Lignocellulose Nanopaper from Agro-Industrial Waste for Green Optoelectronics. ACS Sustain. Chem. Eng. 2020, 8, 17508–17519. [Google Scholar] [CrossRef]
- Cheng, B.-X.; Gao, W.-C.; Ren, X.-M.; Ouyang, X.-Y.; Zhao, Y.; Zhao, H.; Wu, W.; Huang, C.-X.; Liu, Y.; Liu, X.-Y.; et al. A review of microphase separation of polyurethane: Characterization and applications. Polym. Test. 2022, 107, 107489. [Google Scholar] [CrossRef]
- Azizi, S.; Shahri, M.M.; Mohamad, R. Green Synthesis of Zinc Oxide Nanoparticles for Enhanced Adsorption of Lead Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef]
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P.R. Lead and Chromium Adsorption from Water using L-Cysteine Functionalized Magnetite (Fe3O4) Nanoparticles. Sci. Rep. 2017, 7, 7672. [Google Scholar] [CrossRef] [Green Version]
- Moon, G.H.; Jung, Y.; Shin, B. On-Chip Chemiresistive Sensor Array for On-Road NO x Monitoring with Quantification. Adv. Sci. 2020, 7, 2002014. [Google Scholar] [CrossRef]
- Pham, T.; Bui, T.T.; Nguyen, V.T.; Van Bui, T.K.; Tran, T.T.; Phan, Q.C.; Hoang, T.H. Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal. Polymers 2018, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, F.; Peng, Z. Adsorption mechanism of Cr(VI) onto GO/PAMAMs composites. Sci. Rep. 2019, 9, 3663. [Google Scholar] [CrossRef] [Green Version]
- Duan, C.; Zhang, Y.; Li, J.; Kang, L.; Xie, Y.; Qiao, W.; Zhu, C.; Luo, H. Rapid Room-Temperature Preparation of Hierarchically Porous Metal–Organic Frameworks for Efficient Uranium Removal from Aqueous Solutions. Nanomaterials 2020, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Zhao, Z.; Li, Z.; Li, Q. The adsorptive behaviour of kaolinite to sodium dodecyl benzene sulphonate and the structural variation of kaolinite. Sci. Rep. 2021, 11, 1796. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta-Schubert, N.; Tiwari, D.K.; Cendejas, L.M.V. Comment on ‘Carbon and fullerene nanomaterials in plant system’. J. Nanobiotechnol. 2016, 14, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raibaut, L.; Cargoët, M.; Ollivier, N.; Chang, Y.M.; Drobecq, H.; Boll, E.; Desmet, R.; Monbaliu, J.-C.M.; Melnyk, O. Accelerating chemoselective peptide bond formation using bis(2-selenylethyl)amido peptide selenoester surrogates. Chem. Sci. 2016, 7, 2657–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Samples | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| K1 (mL mg−1) | R2 | Qm (mg g−1) | K2 (mL mg−1) | R2 | 1/n | |
| Bi-MIPs | 0.1370 | 0.9953 | 209.64 | 34.20 | 0.9669 | 1.106 |
| Bi-NIPs | 0.1935 | 0.9946 | 106.72 | 25.77 | 0.9788 | 1.116 |
| Si-MIPs | 0.1193 | 0.9912 | 60.350 | 30.20 | 0.9736 | 1.183 |
| Si-NIPs | 0.3417 | 0.9905 | 176.99 | 23.75 | 0.9596 | 1.046 |
| Samples | PFO Kinetic | PSO Kinetic | ||
|---|---|---|---|---|
| K3 (min−1) | R2 | K4 (g mg−1 min−1) | R2 | |
| Bi-MIPs | 0.0533 | 0.9153 | 0.00015 | 0.9903 |
| Bi-NIPs | 0.0421 | 0.9746 | 0.00023 | 0.9816 |
| Si-MIPs | 0.0466 | 0.9751 | 0.00020 | 0.9841 |
| Si-NIPs | 0.0275 | 0.9750 | 0.00026 | 0.9808 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, L.; Cheng, G.; Wei, S.; Wang, Y.; Huang, Q.; Wu, W.; Liu, X.; Chen, G. Study of the Preparation and Properties of Chrysin Binary Functional Monomer Molecularly Imprinted Polymers. Polymers 2022, 14, 2771. https://doi.org/10.3390/polym14142771
Li L, Li L, Cheng G, Wei S, Wang Y, Huang Q, Wu W, Liu X, Chen G. Study of the Preparation and Properties of Chrysin Binary Functional Monomer Molecularly Imprinted Polymers. Polymers. 2022; 14(14):2771. https://doi.org/10.3390/polym14142771
Chicago/Turabian StyleLi, Long, Lanfu Li, Gege Cheng, Sentao Wei, Yaohui Wang, Qin Huang, Wei Wu, Xiuyu Liu, and Guoning Chen. 2022. "Study of the Preparation and Properties of Chrysin Binary Functional Monomer Molecularly Imprinted Polymers" Polymers 14, no. 14: 2771. https://doi.org/10.3390/polym14142771