Sandwich-Structured Flexible PVA/CS@MWCNTs Composite Films with High Thermal Conductivity and Excellent Electrical Insulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of CS@MWCNTs
2.3. Preparation of PVA/Glycerol Solution
2.4. Preparation of PVA/CS@MWCNTs Composite Films
2.5. Characterization
3. Results and Discussions
3.1. Chitosan Coating on the Surface of Carbon Nanotubes
3.2. Dispersion and Interaction of CS@MWCNTs in PVA Matrix
3.3. Microstructure of the Prepared PVA/CS@MWCNTs Composite Films
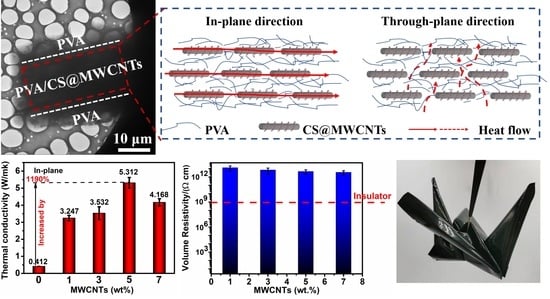
3.4. Thermal Conductivity Properties and Analysis of PVA/CS@MWCNTs Composite Films
3.5. Electrical Insulating Properties and Flexibility Demonstration of PVA/CS@MWCNTs Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xu, B.; Hu, S.; Hung, S.-W.; Shao, C.; Chandra, H.; Chen, F.-R.; Kodama, T.; Shiomi, J. Weaker bonding can give larger thermal conductance at highly mismatched interfaces. Sci. Adv. 2021, 7, eabf8197. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Qin, Z.; Wu, H.; Li, M.; Hu, Y. Flexible thermal interface based on self-assembled boron arsenide for high-performance thermal management. Nat. Commun. 2021, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kraemer, D.; Song, B.; Jiang, Z.; Zhou, J.; Loomis, J.; Wang, J.; Li, M.; Ghasemi, H.; Huang, X.; et al. Nanostructured polymer films with metal-like thermal conductivity. Nat. Commun. 2019, 10, 1771. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose Nanofiber Supported 3D Interconnected BN Nanosheets for Epoxy Nanocom-posites with Ultrahigh Thermal Management Capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.M.; Chang, Y.H. Water-induced shape memory behavior of poly (vinyl alcohol) and p-coumaric ac-id-modified water-soluble chitosan blended membrane. Carbohyd. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Biswal, A.K.; Chang, Y.-H.; Misra, P.K.; Yang, J.-M. Synthesis and Characterization of Modified Poly(vinyl alcohol) Membrane and Study of Its Enhanced Water-Induced Shape-Memory Behavior. J. Polym. Environ. 2022, 30, 1–11. [Google Scholar] [CrossRef]
- Suhrenbrock, L.; Radtke, G.; Knop, K.; Kleinebudde, P. Suspension pellet layering using PVA–PEG graft copolymer as a new binder. Int. J. Pharm. 2011, 412, 28–36. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Kuang, Z.; Chen, Y.; Lu, Y.; Liu, L.; Hu, S.; Wen, S.; Mao, Y.; Zhang, L. Fabrication of Highly Oriented Hexagonal Boron Nitride Nanosheet/Elastomer Nanocomposites with High Thermal Conductivity. Small 2014, 11, 1655–1659. [Google Scholar] [CrossRef]
- Cui, X.; Ding, P.; Zhuang, N.; Shi, L.; Song, N.; Tang, S. Thermal Conductive and Mechanical properties of Polymeric Composites Based on Solution-Exfoliated Boron Nitride and Graphene PPNanosheets: A Morphology-Promoted Synergistic Effect. ACS Appl. Mater. Inter. 2015, 7, 19068–19075. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Geng, H.; Song, X.; Zeng, H.; Zhi, C. Highly Flexible and Self-Healable Thermal Interface Material Based on Boron Nitride Nanosheets and a Dual Cross-Linked Hydrogel. ACS Appl. Mater. Interfaces 2017, 9, 10078–10084. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qi, G.-Q.; Tang, L.-S.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Novel photodriven composite phase change materials with bioinspired modification of BN for solar-thermal energy conversion and storage. J. Mater. Chem. A 2016, 4, 9625–9634. [Google Scholar] [CrossRef]
- Hu, L.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Flexible and thermal conductive poly (vinylidene fluoride) composites with silver decorated hexagonal boron nitride/silicon carbide hybrid filler. Polym. Compos. 2022, 43, 3960–3970. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, D.; Wu, L. Improved thermal conductivity of ceramic-epoxy composites by constructing vertically aligned nanoflower-like AlN network. Ceram. Int. 2021, 48, 10438–10446. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, K.; Liu, Z.; Wang, M.; Chee, K.W.A.; Lin, C.-T.; Jiang, N.; Yu, J. Effective thermal transport highway construction within dielectric polymer composites via a vacuum-assisted infiltration method. J. Mater. Chem. C 2018, 6, 6494–6501. [Google Scholar] [CrossRef]
- Xie, B.-H.; Huang, X.; Zhang, G.-J. High thermal conductive polyvinyl alcohol composites with hexagonal boron nitride micro-platelets as fillers. Compos. Sci. Technol. 2013, 85, 98–103. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Miao, T.; Zhang, X.; Yang, L.; Cai, A.; Yong, Z.; Li, Q. Thermal performance of vertically-aligned multi-walled carbon nanotube array grown on platinum film. Carbon 2014, 77, 266–274. [Google Scholar] [CrossRef]
- Miao, T.; Ma, W.; Zhang, X.; Wei, J.; Sun, J. Significantly enhanced thermoelectric properties of ultralong double-walled carbon nanotube bundle. Appl. Phys. Lett. 2013, 102, 053105. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2014, 21, 11–25. [Google Scholar] [CrossRef]
- Morishita, T.; Matsushita, M. Ultra-highly electrically insulating carbon materials and their use for thermally conductive and electrically insulating polymer composites. Carbon 2021, 184, 786–798. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Guo, Y.; Li, P.; Gao, C. Ultrahigh Thermal Conductive yet Superflexible Graphene Films. Adv. Mater. 2017, 29, 1700589. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Guo, Y.; Song, L.; Hu, Y. Poly(vinyl alcohol) nanocomposites based on graphene and graphite oxide: A comparative investigation of property and mechanism. J. Mater. Chem. 2011, 21, 13942–13950. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Nofal, M.M.; Abdulwahid, R.T.; Hussen, S.A.; Hussein, A.M.; Karim, W.O. A Comprehensive Review on Optical Properties of Polymer Electrolytes and Composites. Materials 2020, 13, 3675. [Google Scholar] [CrossRef]
- Wang, S.-F.; Shen, L.; Zhang, W.-D.; Tong, Y.-J. Preparation and Mechanical Properties of Chitosan/Carbon Nanotubes Composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef]
- Kim, D.; Dhand, V.; Rhee, K.; Park, S.-J. Study on the Effect of Silanization and Improvement in the Tensile Behavior of Gra-phene-Chitosan-Composite. Polymers 2015, 7, 527–551. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Yang, Y.; Wang, L.; Lu, S.; Shao, Z.; Chen, X. Synergistic interactions during thermosensitive chitosan-β-glycerophosphate hydrogel formation. RSC Adv. 2011, 1, 282–289. [Google Scholar] [CrossRef]
- Srinivasa, P.; Ramesh, M.; Kumar, K.; Tharanathan, R. Properties and sorption studies of chitosan–polyvinyl alcohol blend films. Carbohydr. Polym. 2003, 53, 431–438. [Google Scholar] [CrossRef]
- Ma, J.; Liu, C.; Li, R.; Wang, J. Properties and structural characterization of chitosan/poly(vinyl alcohol)/graphene oxide nano composites. e-Poiymers 2012, 12, 033. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Li, T.; Zhao, H.; Li, X.; Gao, C.; Zhang, S.; Xie, E. Graphene-based composite materials beneficial to wound healing. Nanoscale 2012, 4, 2978–2982. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zhang, M.; Chen, S.; Xu, J.; Ma, C.; Chen, G. Sandwich-structured PVA/rGO films from self-construction with high thermal conductivity and electrical insulation. Compos. Sci. Technol. 2021, 207, 108707. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-Level Dispersion of Graphene into Poly(vinyl alcohol) and Effective Reinforcement of their Nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Mahanandia, P.; Vishwakarma, P.; Nanda, K.; Prasad, V.; Subramanyam, S.; Dev, S.; Satyam, P. Multiwall carbon nanotubes from pyrolysis of tetrahydrofuran. Mater. Res. Bull. 2006, 41, 2311–2317. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.; Toh, R.; Hanafiah, M.A.K.M. Comparative study on adsorption and desorption of Cu(II) ions by three types of chitosan–zeolite composites. Chem. Eng. J. 2013, 223, 231–238. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Yang, J.-M.; Chang, Y.-H. Development of chitosan, graphene oxide, and cerium oxide composite blended films: Structural, physical, and functional properties. Cellulose 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, Y.-Y.; Gao, Q.; Luo, W.-J.; Xia, H.; Zhou, C.-G. Highly Efficient Removal of Acid Red 18 from Aqueous Solution by Magnetically Retrievable Chitosan/Carbon Nanotube: Batch Study, Isotherms, Kinetics, and Thermodynamics. J. Chem. Eng. Data 2013, 59, 39–51. [Google Scholar] [CrossRef]
- Cui, L.; Xiong, Z.; Guo, Y.; Liu, Y.; Zhao, J.; Zhang, C.; Zhu, P. Fabrication of interpenetrating polymer network chitosan/gelatin porous materials and study on dye adsorption properties. Carbohydr. Polym. 2015, 132, 330–337. [Google Scholar] [CrossRef]
- Wang, S.; Shen, L.; Tong, Y.; Chen, L.; Phang, I.; Lim, P.; Liu, T. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martínez, G.; Gómez, M.A. Synthesis of poly(vinyl alcohol)/reduced graphite oxide nanocomposites with improved thermal and electrical properties. J. Mater. Chem. 2009, 19, 5027–5032. [Google Scholar] [CrossRef]
- Masser, K.A.; Zhao, H.; Painter, P.C.; Runt, J. Local Relaxation Behavior and Dynamic Fragility in Hydrogen Bonded Polymer Blends. Macromolecules 2010, 43, 9004–9013. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, X.-P.; Wu, J.-L.; Shen, K.-C. Preparation and characterization of graphene/poly(vinyl alcohol) nanocomposites. J. Appl. Polym. Sci. 2010, 118, 275–279. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Wu, H. Preparation and properties of poly(vinyl alcohol)/exfoliated alpha-zirconium phosphate nanocomposite films. Polym. Test. 2009, 28, 371–377. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Shang, S.; Tao, X.-M. Synthesis and characterization of layer-aligned poly(vinyl alcohol)/graphene nanocomposites. Polymer 2010, 51, 3431–3435. [Google Scholar] [CrossRef]
- Lu, L.; Sun, H.; Peng, F.; Jiang, Z. Novel graphite-filled PVA/CS hybrid membrane for pervaporation of benzene/cyclohexane mixtures. J. Membr. Sci. 2006, 281, 245–252. [Google Scholar] [CrossRef]
- Ma, M.; Xu, L.; Qiao, L.; Chen, S.; Shi, Y.; He, H.; Wang, X. Nanofibrillated Cellulose/MgO@rGO composite films with highly an-isotropic thermal conductivity and electrical insulation. Chem. Eng. J. 2020, 392, 123714. [Google Scholar] [CrossRef]
- Choi, J.R.; Yu, S.; Jung, H.; Hwang, S.K.; Kim, R.H.; Song, G.; Cho, S.H.; Bae, I.; Hong, S.M.; Koo, C.M.; et al. Self-assembled block copolymer micelles with silver–carbon nanotube hybrid fillers for high performance thermal conduction. Nanoscale 2014, 7, 1888–1895. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Deng, H.; Jing, Y.; Fu, Q. Enhanced thermal conductivity and electrical insulation properties of polymer com-posites via constructing Pglass/CNTs confined hybrid fillers. Compos. Part A Appl. Sci. Manuf. 2018, 115, 1–7. [Google Scholar] [CrossRef]
- Morishita, T.; Katagiri, Y.; Matsunaga, T.; Muraoka, Y.; Fukumori, K. Design and fabrication of morphologically controlled carbon nanotube/polyamide-6-based composites as electrically insulating materials having enhanced thermal conductivity and elastic modulus. Compos. Sci. Technol. 2017, 142, 41–49. [Google Scholar] [CrossRef]
- Morishita, T.; Matsushita, M.; Katagiri, Y.; Fukumori, K. A novel morphological model for carbon nanotube/polymer composites having high thermal conductivity and electrical insulation. J. Mater. Chem. 2011, 21, 5610–5614. [Google Scholar] [CrossRef]
- Zhang, P.; Ding, X.; Wang, Y.; Gong, Y.; Zheng, K.; Chen, L.; Tian, X.; Zhang, X. Segregated double network enabled effective electromagnetic shielding composites with extraordinary electrical insulation and thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2018, 117, 56–64. [Google Scholar] [CrossRef]
- Zhan, Y.; Lago, E.; Santillo, C.; Castillo, A.E.E.D.R.; Hao, S.; Buonocore, G.G.; Chen, Z.; Xia, H.; Lavorgna, M.; Bonaccorso, F. An anisotropic layer-by-layer carbon nanotube/boron nitride/rubber composite and its application in electromagnetic shielding. Nanoscale 2020, 12, 7782–7791. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, P. Fluorinated Carbon Nanotube/Nanofibrillated Cellulose Composite Film with Enhanced Toughness, Superior Thermal Conductivity, and Electrical Insulation. ACS Appl. Mater. Interfaces 2018, 10, 34311–34321. [Google Scholar] [CrossRef]








| Filler | Filler Loading | Matrix | κ (W·m−1·K−1) | Volume Resistivity (Ω·cm) | Refs. |
|---|---|---|---|---|---|
| MWCNTs | 19.3 vol% | PS-b-P4VP | 0.73 | - | [49] |
| MWCNTs | 3.5 wt% | PP | 0.87 | - | [50] |
| MWCNTs | 1 vol% | PA6 | 0.352 | 1.0 × 1013 | [51] |
| MWCNTs | 2 vol% | PPS/PE/EGMA | 0.57 | 1.9 × 1015 | [52] |
| MWCNTs | 5 wt% | PVDF | 0.83 | 1.2 × 1013 | [53] |
| MWCNTs | 7.4 wt% | NR | 0.25 | - | [54] |
| MWCNTs | 35 wt% | NFCs | 14.1 | 1010 | [55] |
| MWCNTs | 5 wt% | PVA | 5.312 | 4.6 × 1012 | This work |
| Samples | Tensile Strength (MPa) | Elongation at Break |
|---|---|---|
| Pure PVA | 17.55 ± 0.62 | 5.14 ± 0.14 |
| PVA/CS@MWCNTs-1 wt% | 17.83 ± 1.50 | 4.26 ± 0.18 |
| PVA/CS@MWCNTs-3 wt% | 19.26 ± 0.31 | 3.21 ± 0.09 |
| PVA/CS@MWCNTs-5 wt% | 22.97 ± 0.14 | 2.70 ± 0.18 |
| PVA/CS@MWCNTs-7 wt% | 14.75 ± 0.83 | 1.56 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, F.; Ma, C.; Tang, Y.; Zhou, L.; Ding, Y.; Chen, G. Sandwich-Structured Flexible PVA/CS@MWCNTs Composite Films with High Thermal Conductivity and Excellent Electrical Insulation. Polymers 2022, 14, 2512. https://doi.org/10.3390/polym14122512
Luo F, Ma C, Tang Y, Zhou L, Ding Y, Chen G. Sandwich-Structured Flexible PVA/CS@MWCNTs Composite Films with High Thermal Conductivity and Excellent Electrical Insulation. Polymers. 2022; 14(12):2512. https://doi.org/10.3390/polym14122512
Chicago/Turabian StyleLuo, Fanghua, Chen Ma, Yuhui Tang, Lintao Zhou, Youpeng Ding, and Guohua Chen. 2022. "Sandwich-Structured Flexible PVA/CS@MWCNTs Composite Films with High Thermal Conductivity and Excellent Electrical Insulation" Polymers 14, no. 12: 2512. https://doi.org/10.3390/polym14122512