Facile Fabrication of Cellulose Nanofibrils/Chitosan Beads as the Potential pH-Sensitive Drug Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
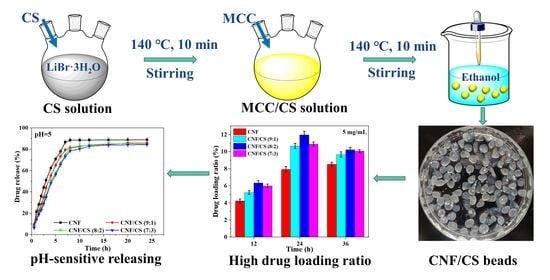
2.2. Preparation of Cellulose Nanofibrils/Chitosan (CNF/CS) Beads
2.3. Characterization
2.4. Swelling and Degradation Properties of CNF/CS Beads
2.5. Drug Loading Capacity of CNF/CS Beads
2.6. Drug Release of CNF/CS Beads In Vitro
3. Results
3.1. Micro-Structure of the Resultant Beads
3.2. Characterization of CNF/CS Beads
3.3. Drug Loading Properties of CNF/CS Beads
3.4. Drug Release Properties of CNF/CS Beads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koklukaya, O.; Carosio, F.; Duran, V.L.; Wagberg, L. Layer-by-layer modified low density cellulose fiber networks: A sustainable and fireproof alternative to petroleum based foams. Carbohydr. Polym. 2020, 230, 115616–115625. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yu, G.; Chen, W.; Dong, S.; Wang, Y.; Liu, C.; Li, B. A pulp foam with highly improved physical strength, fire-resistance and antibiosis by incorporation of chitosan and CPAM. Carbohydr. Polym. 2022, 278, 118963. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced functional materials based on nanocellulose for pharmaceutical/medical applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, C.; He, X.; Zhang, X.; Zhang, W.; Zhang, X. Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte three layer nanoparticles of chitosan/dextran sulfate/chitosan for dual drug delivery. Colloid Surf. B 2020, 190, 110925. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles modified by polydopamine: Working as "drug" carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Zheng, H.; Li, S.; Zhu, L.M. A novel chitosan-based nanomedicine for multi-drug resistant breast cancer therapy. Chem. Eng. J. 2019, 369, 134–149. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-demand dissolvable self-healing hydrogels based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Kimura, S.; Wada, M. Highly enhanced adsorption of Congo red onto dialdehyde cellulose-crosslinked cellulose-chitosan foam. Carbohydr. Polym. 2019, 214, 294–302. [Google Scholar] [CrossRef]
- Yu, S.; Sun, J.; Shi, Y.; Wang, Q.; Wu, J.; Liu, J. Nanocellulose from various biomass wastes: Its preparation and potential usages towards the high value-added products. Environ. Sci. Ecotechnol. 2021, 5, 100077–100090. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, C.; Feng, X.; Wu, M.; Tang, Y.; Li, B. Effect of regeneration solvent on the characteristics of regenerated cellulose from lithium bromide trihydrate molten salt. Cellulose 2020, 27, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Wang, Y.C.; Zhang, S.; Yang, W.; Pan, X.; Wang, Z.L. Cellulose II aerogel-based triboelectric nanogenerator. Adv. Funct. Mater. 2020, 30, 2001763. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, L.; Tian, Y.; Song, J.; Ma, J.; Wang, Z. Rapid dissolution of cellulose in an AlCl3/ZnCl2 aqueous system at room temperature and its versatile adaptability in functional materials. Green Chem. 2022, 24, 885–897. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, X.F.; Wang, Z.; Yao, J. Structure reorganization of cellulose hydrogel by green solvent exchange for potential plastic replacement. Carbohydr. Polym. 2022, 275, 118695. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bang, J.; Kim, J.; Choi, J.H.; Hwang, S.W.; Yeo, H.; Choi, I.G.; Jin, H.J.; Kwak, H.W. Cationic surface-modified regenerated nanocellulose hydrogel for efficient Cr(VI) remediation. Carbohydr. Polym. 2022, 278, 118930. [Google Scholar] [CrossRef]
- Liao, Y.; Pan, X. Self-indicating and high-capacity mesoporous aerogel-based biosorbent fabricated from cellulose and chitosan via co-dissolution and regeneration for removing formaldehyde from indoor air. Environ. Sci. Nano 2021, 8, 1283–1295. [Google Scholar] [CrossRef]
- Mohanta, V.; Madras, G.; Patil, S. Layer-by-layer assembled thin films and microcapsules of nanocrystalline cellulose for hydrophobic drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 20093–20101. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E. FTIR spectra of pseudo-binary sodium borate glasses containing TeO2. J. Mol. Struct. 2012, 1014, 1–6. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Togawa, E.; Kondo, T. Characterization of individual hydrogen bonds in crystalline regenerated cellulose using resolved polarized FTIR spectra. ACS Omega 2017, 2, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, N.; Wang, H.; Liu, C.; Pan, X. Preparation and characterization of regenerated cellulose film from a solution in lithium bromide molten salt hydrate. Polymers 2018, 10, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogilevskaya, E.L.; Akopova, T.A.; Zelenetskii, A.N.; Ozerin, A.N. The crystal structure of chitin and chitosan. Polym. Sci. Ser. A 2006, 48, 116–123. [Google Scholar] [CrossRef]
- Gözaydın, G.; Song, S.; Yan, N. Chitin hydrolysis in acidified molten salt hydrates. Green Chem. 2020, 22, 5096–5104. [Google Scholar] [CrossRef]
- Lindström, T.; Fellers, C.; Ankerfors, M.; Nordmark, G.G. On the nature of joint strength of paper—Effect of dry strength agents—Revisiting the Page equation. Nord. Pulp Pap. Res. J. 2018, 31, 459–468. [Google Scholar] [CrossRef]
- Yang, S.C.; Liao, Y.; Karthikeyan, K.G.; Pan, X.J. Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ. Pollut. 2021, 286, 117324. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kurki Suonio, S.; Wagermaier, W.; Hynninen, V.; Hietala, S.; Ikkala, O. Interfacial polyelectrolyte complex spinning of cellulose nanofibrils for advanced bicomponent fibers. Biomacromolecules 2017, 18, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Chen, M.J.; Zhang, X.Q.; Liu, C.F.; Sun, R.C. Per-O-acetylation of cellulose in dimethyl sulfoxide with catalyzed transesterification. J. Agric. Food. Chem. 2014, 62, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yuan, Q.; Yang, M.; Liu, R.; Zhu, S.; Li, J.; Zhang, W.; You, J.; Xiong, S.; Hu, Y. Development of biocompatible and antibacterial collagen hydrogels via dialdehyde polysaccharide modification and tetracycline hydrochloride loading. Macromol. Mater. Eng. 2019, 304, 1800755. [Google Scholar] [CrossRef]
- Xu, S.; Zeng, R.; Cheng, J.; Cai, Z.; Wen, X.; Pi, P. Preparation of antimicrobial polycarboxybetaine-based hydrogels for studies of drug loading and release. J. Appl. Polym. Sci. 2014, 131, 39839. [Google Scholar] [CrossRef]
- Nam, H.S.; An, J.; Chung, D.J.; Kim, J.H.; Chung, C.P. Controlled release behavior of bioactive molecules from photo-reactive hyaluronic acid-alginate scaffolds. Macromol. Res. 2006, 14, 530–538. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Saputra, S.N.; Soetaredjo, F.E.; Putro, J.N.; Lin, C.X.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydr. Polym. 2017, 175, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fan, Q.; Huo, Y.; Liu, C.; Li, B.; Li, Y. Construction of a mesoporous polydopamine@GO/Cellulose nanofibril composite hydrogel with an encapsulation structure for controllable drug release and toxicity shielding. ACS Appl. Mater. Interfaces 2020, 12, 57410–57420. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Ajdnik, U.; Nagaraj, C.; Lackner, F.; Dobaj Stiglic, A.; Palani, T.; Amornkitbamrung, L.; Gradisnik, L.; Maver, U.; Kargl, R.; et al. One-step fabrication of hollow spherical cellulose beads: Application in ph-responsive therapeutic delivery. ACS Appl. Mater. Interfaces 2022, 14, 3726–3739. [Google Scholar] [CrossRef]






| Sample | Nanofiber Diameter (nm) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| CNF beads | 31.8 ± 6.0 | 3.2 ± 0.1 | 0.0122 ± 0.012 | 17.5 ± 1.1 |
| CNF/CS beads (9:1) | 16.5 ± 3.1 | 9.2 ± 0.1 | 0.0299 ± 0.009 | 13.3 ± 1.3 |
| CNF/CS beads (8:2) | 21.3 ± 3.4 | 17.9 ± 0.2 | 0.0541 ± 0.010 | 12.2 ± 1.2 |
| CNF/CS beads (7:3) | 24.7 ± 5.2 | 11.3 ± 0.1 | 0.0540 ± 0.011 | 16.0 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Deng, W.; Zhang, Y.; Chen, C.; Liu, Z.; Fatehi, P.; Li, B. Facile Fabrication of Cellulose Nanofibrils/Chitosan Beads as the Potential pH-Sensitive Drug Carriers. Polymers 2022, 14, 2286. https://doi.org/10.3390/polym14112286
Wu M, Deng W, Zhang Y, Chen C, Liu Z, Fatehi P, Li B. Facile Fabrication of Cellulose Nanofibrils/Chitosan Beads as the Potential pH-Sensitive Drug Carriers. Polymers. 2022; 14(11):2286. https://doi.org/10.3390/polym14112286
Chicago/Turabian StyleWu, Meiyan, Wangfang Deng, Yidong Zhang, Chao Chen, Zhexuan Liu, Pedram Fatehi, and Bin Li. 2022. "Facile Fabrication of Cellulose Nanofibrils/Chitosan Beads as the Potential pH-Sensitive Drug Carriers" Polymers 14, no. 11: 2286. https://doi.org/10.3390/polym14112286