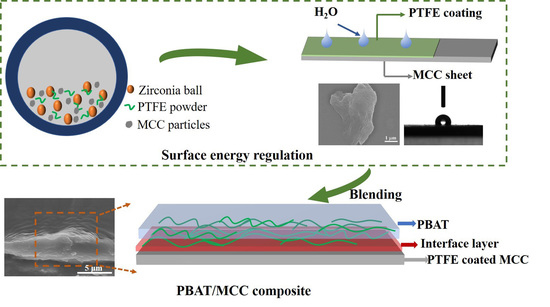
Tailoring Interfacial Adhesion between PBAT Matrix and PTFE-Modified Microcrystalline Cellulose Additive for Advanced Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Obtaining of Hydrophobic Coating on MCC Surface
2.3. The Preparation of PBAT/N#MCC Composites
2.4. Characterization
3. Results and Discussion
3.1. Morphology of PTFE-Coated MCC
3.2. Thermal Behavior of PTFE-Coated MCC
3.3. Wetting Properties of PTFE-Coated MCC
3.4. Mechanical Properties of PBAT/N#MCC Composites
3.5. The Interface Adhesion between PBAT Matrix and PTFE-Modified MCC
3.6. Thermal Behavior of PBAT/N#MCC Composites
3.7. Viscoelastic Properties of PBAT/N#MCC Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Shen, J.; Chen, S.; Cooper, M.A.; Fu, H.; Wu, D.; Yang, Z. Nanofiller Reinforced Biodegradable PLA/PHA Composites: Current Status and Future Trends. Polymers 2018, 10, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Sereda, A.; Gaidukova, G.; Grase, L.; Thakur, V.K.; Filipova, I.; Fridrihsone, V.; Skute, M. Bio-Based Poly(butylene succinate)/Microcrystalline Cellulose/Nanofibrillated Cellulose-Based Sustainable Polymer Composites: Thermo-Mechanical and Biodegradation Studies. Polymers 2020, 12, 1472. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Stepanova, M.; Korzhikova-Vlakh, E. Modification of Cellulose Micro- and Nanomaterials to Improve Properties of Aliphatic Polyesters/Cellulose Composites: A Review. Polymers 2022, 14, 1477. [Google Scholar] [CrossRef]
- Zhou, L.; Ke, K.; Yang, M.-B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly(lactic acid)/Cellulose composite: A review. Compos. Commun. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, L.; Jiang, X.; Wang, H.; Gao, W. Cellulose nanocrystals modified with a triazine derivative and their reinforcement of poly(lactic acid)-based bionanocomposites. Cellulose 2018, 25, 2965–2976. [Google Scholar] [CrossRef]
- Lo Re, G.; Engström, J.; Wu, Q.; Malmström, E.; Gedde, U.W.; Olsson, R.T.; Berglund, L. Improved Cellulose Nanofibril Dispersion in Melt-Processed Polycaprolactone Nanocomposites by a Latex-Mediated Interphase and Wet Feeding as LDPE Alternative. ACS Appl. Nano Mater. 2018, 1, 2669–2677. [Google Scholar] [CrossRef]
- Singh, A.A.; Geng, S.; Herrera, N.; Oksman, K. Aligned plasticized polylactic acid cellulose nanocomposite tapes: Effect of drawing conditions. Compos. Part A 2018, 104, 101–107. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, G.; Lu, X.-a.; Wang, J.; Wu, D. Cellulosic nanofibers filled poly(β-hydroxybutyrate): Relations between viscoelasticity of composites and aspect ratios of nanofibers. Carbohydr. Polym. 2021, 265, 118093–118102. [Google Scholar] [CrossRef]
- Follain, N.; Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Marais, S.; Dufresne, A. Tunable gas barrier properties of filled-PCL film by forming percolating cellulose network. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 26–30. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; He, M.; Jiang, Q.; Li, A.; Li, S.; Liu, M.; Luo, S.; Zhang, D. Improvement of polylactic acid film properties through the addition of cellulose nanocrystals isolated from waste cotton cloth. Int. J. Biol. Macromol. 2019, 129, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Gazzotti, S.; Rampazzo, R.; Hakkarainen, M.; Bussini, D.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. Cellulose nanofibrils as reinforcing agents for PLA-based nanocomposites: An in situ approach. Compos. Sci. Technol. 2019, 171, 94–102. [Google Scholar] [CrossRef]
- Sakakibara, K.; Moriki, Y.; Yano, H.; Tsujii, Y. Strategy for the Improvement of the Mechanical Properties of Cellulose Nanofiber-Reinforced High-Density Polyethylene Nanocomposites Using Diblock Copolymer Dispersants. ACS Appl. Mater. Interfaces 2017, 9, 44079–44087. [Google Scholar] [CrossRef]
- Sakakibara, K.; Yano, H.; Tsujii, Y. Surface Engineering of Cellulose Nanofiber by Adsorption of Diblock Copolymer Dispersant for Green Nanocomposite Materials. ACS Appl. Mater. Interfaces 2016, 8, 24893–24900. [Google Scholar] [CrossRef]
- Chai, H.; Chang, Y.; Zhang, Y.; Chen, Z.; Zhong, Y.; Zhang, L.; Sui, X.; Xu, H.; Mao, Z. The fabrication of polylactide/cellulose nanocomposites with enhanced crystallization and mechanical properties. Int. J. Biol. Macromol. 2020, 155, 1578–1588. [Google Scholar] [CrossRef]
- Lafia-Araga, R.A.; Sabo, R.; Nabinejad, O.; Matuana, L.; Stark, N. Influence of Lactic Acid Surface Modification of Cellulose Nanofibrils on the Properties of Cellulose Nanofibril Films and Cellulose Nanofibril-Poly(lactic acid) Composites. Biomolecules 2021, 11, 1346. [Google Scholar] [CrossRef]
- Simão, J.A.; Bellani, C.F.; Branciforti, M.C. Thermal properties and crystallinity of PCL/PBSA/cellulose nanocrystals grafted with PCL chains. J. Appl. Polym. Sci. 2017, 134, 44493. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Reinforcement effect of poly(butylene succinate) (PBS)-grafted cellulose nanocrystal on toughened PBS/polylactic acid blends. Carbohydr. Polym. 2016, 140, 374–382. [Google Scholar] [CrossRef]
- Wågberg, L.; Decher, G.; Norgren, M.; Lindström, T.; Ankerfors, M.; Axnäs, K. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Salajková, M.; Berglund, L.A.; Zhou, Q. Hydrophobic cellulose nanocrystals modified with quaternary ammonium salts. J. Mater. Chem. 2012, 22, 19798–19805. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, Z.; Wang, C.; Wang, A.; Liu, X.; Wei, B.; Wen, Y. Enhancing the redispersibility of TEMPO-mediated oxidized cellulose nanofibrils in N,N-dimethylformamide by modification with cetyltrimethylammonium bromide. Cellulose 2019, 26, 7769–7780. [Google Scholar] [CrossRef]
- Nair, S.S.; Chen, H.; Peng, Y.; Huang, Y.; Yan, N. Polylactic Acid Biocomposites Reinforced with Nanocellulose Fibrils with High Lignin Content for Improved Mechanical, Thermal, and Barrier Properties. ACS Sustain. Chem. Eng. 2018, 6, 10058–10068. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef] [Green Version]
- Kuga, S.; Wu, M. Mechanochemistry of cellulose. Cellulose 2019, 26, 215–225. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Q.; Wang, Q.; Wolcott, M. One-Step Activation and Surface Fatty Acylation of Cellulose Fibers in a Solvent-Free Condition. ACS Sustain. Chem. Eng. 2019, 7, 15920–15927. [Google Scholar] [CrossRef]
- Lang, H.; Qiong, W.; Wang, Q.; Ou, R.; Wolcott, M. Solvent-free pulverization and surface fatty acylation of pulp fiber for property-enhanced cellulose/polypropylene composites. J. Clean. Prod. 2020, 244, 118811–118818. [Google Scholar] [CrossRef]
- Huang, P.; Zhao, Y.; Kuga, S.; Wu, M.; Huang, Y. A versatile method for producing functionalized cellulose nanofibers and their application. Nanoscale 2016, 8, 3753–3759. [Google Scholar] [CrossRef]
- Zhao, M.M.; Kuga, S.; Jiang, S.D.; Wu, M.; Huang, Y. Cellulose nanosheets induced by mechanical impacts under hydrophobic environment. Cellulose 2016, 23, 2809–2818. [Google Scholar] [CrossRef]
- Zhao, M.M.; Kuga, S.; Wu, M.; Huang, Y. Hydrophobic nanocoating of cellulose by solventless mechanical milling. Green Chem. 2016, 18, 3006–3012. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, J.; Ye, H.; Dong, Y.; Zhou, Q. Combined effect of cellulose nanocrystals and poly(butylene succinate) on poly(lactic acid) crystallization: The role of interfacial affinity. Carbohydr. Polym. 2018, 179, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.d.; Kano, F.S.; Rosa, D.d.S. Thermal stability of the PBAT biofilms with cellulose nanostructures/essential oils for active packaging. J. Therm. Anal. Calorim. 2019, 138, 2375–2386. [Google Scholar] [CrossRef]
- Lewin, M.; Mey-Marom, A.; Frank, R. Surface free energies of polymeric materials, additivesand minerals. Polym. Adv. Technol. 2005, 16, 429–441. [Google Scholar] [CrossRef]
- Botta, L.; Titone, V.; Mistretta, M.C.; La Mantia, F.P.; Modica, A.; Bruno, M.; Sottile, F.; Lopresti, F. PBAT Based Composites Reinforced with Microcrystalline Cellulose Obtained from Softwood Almond Shells. Polymers 2021, 13, 2643. [Google Scholar] [CrossRef]
- Peterson, A.; Mehandzhiyski, A.Y.; Svenningsson, L.; Ziolkowska, A.; Kádár, R.; Lund, A.; Sandblad, L.; Evenäs, L.; Lo Re, G.; Zozoulenko, I.; et al. A Combined Theoretical and Experimental Study of the Polymer Matrix-Mediated Stress Transfer in a Cellulose Nanocomposite. Macromolecules 2021, 54, 3507–3516. [Google Scholar] [CrossRef]
- Lugscheider, E.; Bobzin, K. The influence on surface free energy of PVD-coatings. Surf. Coat. Technol. 2001, 142, 755–760. [Google Scholar] [CrossRef]
- Xu, C.; Wu, D.; Lv, Q.; Yan, L. Crystallization Temperature as the Probe To Detect Polymer–Filler Compatibility in the Poly(ε-caprolactone) Composites with Acetylated Cellulose Nanocrystal. J. Phys. Chem. C 2017, 121, 18615–18624. [Google Scholar] [CrossRef]
- Zhongzhen, Y.; Yuchun, O.; Pengyu, F. Effect of interfacial adhesion on crystallization and mechanical properties of polyethylene terephthalate/polyamide blends. Polym. Mater. Sci. Eng. 1997, 13, 57–61. [Google Scholar] [CrossRef]
- Yin, Y.; Ma, J.; Tian, X.; Jiang, X.; Wang, H.; Gao, W. Cellulose nanocrystals functionalized with amino-silane and epoxy-poly(ethylene glycol) for reinforcement and flexibilization of poly(lactic acid): Material preparation and compatibility mechanism. Cellulose 2018, 25, 6447–6463. [Google Scholar] [CrossRef]
- Hu, Y.S.; Prattipati, V.; Mehta, S.; Schiraldi, D.A.; Hiltner, A.; Baer, E. Improving gas barrier of PET by blending with aromatic polyamides. Polymer 2005, 46, 2685–2698. [Google Scholar] [CrossRef]









| Sample | CA of H2O/° | CA of DMF/° | γ (mJ/m2) | γd (mJ/m2) | γp (mJ/m2) | xd | xp |
|---|---|---|---|---|---|---|---|
| Water | —— | —— | 72.80 | 29.10 | 43.70 | 0.400 | 0.600 |
| N,N-Dimethylformamide | —— | —— | 37.30 | 32.42 | 4.88 | 0.869 | 0.131 |
| MCC | 34.2 | 8.8 | 64.27 | 12.08 | 52.19 | 0.188 | 0.812 |
| PBAT | 69.5 | 12.1 | 35.54 | 27.67 | 9.87 | 0.779 | 0.211 |
| 0.2%PTFE-MCC | 38.6 | 25.8 | 60.43 | 12.52 | 47.91 | 0.207 | 0.793 |
| 0.5%PTFE-MCC | 69.1 | 29.3 | 35.44 | 22.36 | 13.08 | 0.631 | 0.369 |
| 1%PTFE-MCC | 75.4 | 50.0 | 29.07 | 15.70 | 13.38 | 0.540 | 0.460 |
| 2%PTFE-MCC | 108.0 | 68.5 | 22.48 | 22.33 | 0.15 | 0.9933 | 0.0067 |
| 4%PTFE-MCC | 115.0 | 73.7 | 18.92 | 18.79 | 0.13 | 0.9931 | 0.0069 |
| PTFE | —— | —— | 18.6 [35] | —— | —— | —— | —— |
| Composites | E1-γ12 (mJ/m2) | E2-γ12 (mJ/m2) | φ |
|---|---|---|---|
| PBAT/0#MCC | 32.97 | 17.85 | 0.797 |
| PBAT/0.2#MCC | 28.77 | 15.25 | 0.811 |
| PBAT/0.5#MCC | −0.94 | −1.49 | 0.980 |
| PBAT/1#MCC | 1.83 | −0.05 | 0.960 |
| PBAT/2#MCC | 8.00 | 5.88 | 0.918 |
| PBAT/4#MCC | 9.19 | 6.26 | 0.918 |
| Composites | Tg/°C | Thc/°C (Melting) | Tcc/°C (Cooling) | Tm/°C | ∆Hm/(J/g) | χc/% | ∆T/°C (Tm-Tcc) |
|---|---|---|---|---|---|---|---|
| PBAT | −30.66 | 89.61 | 77.11 | 121.75 | 11.77 | 10.32 | 44.64 |
| PBAT/0#MCC | −31.02 | 91.61 | 80.27 | 123.08 | 10.22 | 8.96 | 42.81 |
| PBAT/0.2#MCC | −31.24 | 94.82 | 84.57 | 123.75 | 9.47 | 8.31 | 39.18 |
| PBAT/0.5#MCC | −31.28 | 97.45 | 86.07 | 124.44 | 9.25 | 8.11 | 38.37 |
| PBAT/1#MCC | −31.45 | 97.15 | 85.85 | 124.57 | 9.16 | 8.03 | 38.72 |
| PBAT/2#MCC | −31.02 | 96.13 | 85.60 | 124.90 | 8.85 | 7.76 | 39.30 |
| PBAT/4#MCC | −31.00 | 95.06 | 85.42 | 124.71 | 8.65 | 7.59 | 39.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, X.; Liu, J.; Wu, M.; Huang, Y. Tailoring Interfacial Adhesion between PBAT Matrix and PTFE-Modified Microcrystalline Cellulose Additive for Advanced Composites. Polymers 2022, 14, 1973. https://doi.org/10.3390/polym14101973
Wang H, Liu X, Liu J, Wu M, Huang Y. Tailoring Interfacial Adhesion between PBAT Matrix and PTFE-Modified Microcrystalline Cellulose Additive for Advanced Composites. Polymers. 2022; 14(10):1973. https://doi.org/10.3390/polym14101973
Chicago/Turabian StyleWang, Hongkun, Xuran Liu, Jinfeng Liu, Min Wu, and Yong Huang. 2022. "Tailoring Interfacial Adhesion between PBAT Matrix and PTFE-Modified Microcrystalline Cellulose Additive for Advanced Composites" Polymers 14, no. 10: 1973. https://doi.org/10.3390/polym14101973