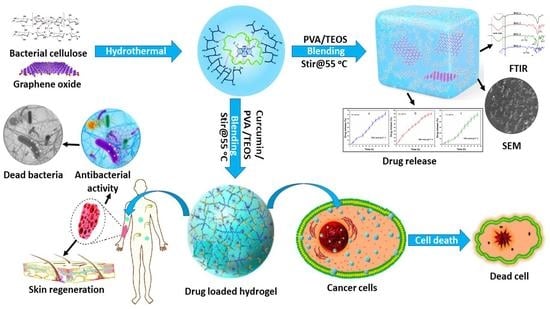
pH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Fabrication
3. Characterizations
3.1. FT-IR
3.2. SEM
3.3. Water Contact Angle
3.4. Swelling
3.5. Biodegradation
3.6. Curcumin Loading and Franz Diffusion Release
3.7. Drug Release Kinetics
3.8. In Vitro Analysis
3.8.1. Antimicrobial Activities
3.8.2. Anticancer Analysis
3.9. Statistical Analysis
4. Results and Discussions
4.1. FTIR Analysis
4.2. SEM Morphology
4.3. Water Contact Angle
4.4. Swelling
4.5. Biodegradation
4.6. Mechanical Testing
4.7. In Vitro Drug Release
4.8. Drug Release Kinetics
4.9. Antimicrobial Activities
4.10. Anticancer Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Z.; Niu, J.; Cheng, B. Prevalence of chronic skin wounds and their risk factors in an inpatient hospital setting in northern China. Adv. Ski. Wound Care 2020, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Aslam Khan, M.U.; Abd Razak, S.I.; Al Arjan, W.S.; Nazir, S.; Sahaya Anand, T.J.; Mehboob, H.; Amin, R. Recent Advances in Biopolymeric Composite Materials for Tissue Engineering and Regenerative Medicines: A Review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Iqbal, I.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Jabeen, F.; Riduan Mohamad, M.; Jusoh, N. Development of Antibacterial, Degradable and pH-Responsive Chitosan/Guar Gum/Polyvinyl Alcohol Blended Hydrogels for Wound Dressing. Molecules 2021, 26, 5937. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razaq, S.I.A.; Mehboob, H.; Rehman, S.; Al-Arjan, W.S.; Amin, R. Antibacterial and hemocompatible pH-responsive hydrogel for skin wound healing application: In vitro drug release. Polymers 2021, 13, 3703. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers 2021, 13, 2529. [Google Scholar] [CrossRef]
- Panduranga Rao, K. Recent developments of collagen-based materials for medical applications and drug delivery systems. J. Biomater. Sci. Polym. Ed. 1996, 7, 623–645. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Al-Arjan, W.S.; Binkadem, M.S.; Mehboob, H.; Haider, A.; Raza, M.A.; Abd Razak, S.I.; Hasan, A.; Amin, R. Development of Biopolymeric Hybrid Scaffold-Based on AAc/GO/nHAp/TiO2 Nanocomposite for Bone Tissue Engineering: In-Vitro Analysis. Nanomaterials 2021, 11, 1319. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, F.; Zhang, Z.; Cheng, Y.; Wang, Z.; Li, Y. Stimuli-responsive polydopamine-based smart materials. Chem. Soc. Rev. 2021, 50, 8319–8343. [Google Scholar] [CrossRef]
- Abruzzo, A.; Cappadone, C.; Sallustio, V.; Picone, G.; Rossi, M.; Nicoletta, F.P.; Luppi, B.; Bigucci, F.; Cerchiara, T. Development of Spanish Broom and Flax Dressings with Glycyrrhetinic Acid-Loaded Films for Wound Healing: Characterization and Evaluation of Biological Properties. Pharmaceutics 2021, 13, 1192. [Google Scholar] [CrossRef] [PubMed]
- Aslam Khan, M.U.; Haider, A.; Abd Razak, S.I.; Abdul Kadir, M.R.; Haider, S.; Shah, S.A.; Hasan, A.; Khan, R.; Khan, S.U.D.; Shakir, I. Arabinoxylan/graphene-oxide/nHAp-NPs/PVA bionano composite scaffolds for fractured bone healing. J. Tissue Eng. Regen. Med. 2021, 15, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; Li, L.; Zhu, F.; Ren, X.; Huang, Q.; Cheng, Y.; Li, Y. Bioinspired integration of naturally occurring molecules towards universal and smart antibacterial coatings. Adv. Funct. Mater. 2022, 32, 2108749. [Google Scholar] [CrossRef]
- Jiji, S.; Udhayakumar, S.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol. 2019, 122, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Amin, M.C.I.M. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Zhuang, W.; Ge, L.; Wang, Z.; Wu, J.; Niu, H.; Liu, D.; Zhu, C.; Chen, Y.; Ying, H. Surface functionalization of graphene oxide by amino acids for Thermomyces lanuginosus lipase adsorption. J. Colloid Interface Sci. 2019, 546, 211–220. [Google Scholar] [CrossRef]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C 2018, 86, 173–197. [Google Scholar] [CrossRef]
- Abba, M.; Ibrahim, Z.; Chong, C.S.; Zawawi, N.A.; Kadir, M.R.A.; Yusof, A.H.M.; Abd Razak, S.I. Transdermal delivery of crocin using bacterial nanocellulose membrane. Fibers Polym. 2019, 20, 2025–2031. [Google Scholar] [CrossRef]
- Nazir, S.; Khan, M.U.A.; Al-Arjan, W.S.; Abd Razak, S.I.; Javed, A.; Kadir, M.R.A. Nanocomposite hydrogels for melanoma skin cancer care and treatment: In-vitro drug delivery, drug release kinetics and anti-cancer activities. Arab. J. Chem. 2021, 14, 103120. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razak, S.I.A.; Ansari, M.N.M.; Zulkifli, R.M.; Ahmad Zawawi, N.; Arshad, M. Development of Biodegradable Bio-Based Composite for Bone Tissue Engineering: Synthesis, Characterization and In Vitro Biocompatible Evaluation. Polymers 2021, 13, 3611. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Karahaliloglu, Z.; Kilicay, E.; Denkbas, E.B. Antibacterial chitosan/silk sericin 3D porous scaffolds as a wound dressing material. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1172–1185. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and copper co-substituted hydroxyapatite-based coatings with improved antibacterial activity and cytocompatibility fabricated by electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Tan, G.; Wang, Y.; Li, J.; Zhang, S. Synthesis and characterization of injectable photocrosslinking poly (ethylene glycol) diacrylate based hydrogels. Polym. Bull. 2008, 61, 91–98. [Google Scholar] [CrossRef]
- Jeddi, M.K.; Mahkam, M. Magnetic nano carboxymethyl cellulose-alginate/chitosan hydrogel beads as biodegradable devices for controlled drug delivery. Int. J. Biol. Macromol. 2019, 135, 829–838. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Raza, M.A.; Razak, S.I.A.; Abdul Kadir, M.R.; Haider, A.; Shah, S.A.; Mohd Yusof, A.H.; Haider, S.; Shakir, I.; Aftab, S. Novel functional antimicrobial and biocompatible arabinoxylan/guar gum hydrogel for skin wound dressing applications. J. Tissue Eng. Regen. Med. 2020, 14, 1488–1501. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Yaqoob, Z.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Haider, S.; Busra, F.M. Chitosan/Poly Vinyl Alcohol/Graphene Oxide Based pH-Responsive Composite Hydrogel Films: Drug Release, Anti-Microbial and Cell Viability Studies. Polymers 2021, 13, 3124. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Raza, M.A.; Shah, S.A.; Abd Razak, S.I.; Kadir, M.R.A.; Subhan, F.; Haider, A. Smart and pH-sensitive rGO/Arabinoxylan/chitosan composite for wound dressing: In-vitro drug delivery, antibacterial activity, and biological activities. Int. J. Biol. Macromol. 2021, 192, 820–831. [Google Scholar] [CrossRef]
- Hong, Y.; Song, H.; Gong, Y.; Mao, Z.; Gao, C.; Shen, J. Covalently crosslinked chitosan hydrogel: Properties of in vitro degradation and chondrocyte encapsulation. Acta Biomater. 2007, 3, 23–31. [Google Scholar] [CrossRef]
- McBath, R.A.; Shipp, D.A. Swelling and degradation of hydrogels synthesized with degradable poly (β-amino ester) crosslinkers. Polym. Chem. 2010, 1, 860–865. [Google Scholar] [CrossRef]
- Son, G.-H.; Lee, B.-J.; Cho, C.-W. Mechanisms of drug release from advanced drug formulations such as polymeric-based drug-delivery systems and lipid nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Mirzaei, B.E.; Ramazani SA, A.; Shafiee, M.; Danaei, M. Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 605–611. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; p. 63. [Google Scholar]
- Khan, M.U.A.; Abd Razak, S.I.; Haider, S.; Mannan, H.A.; Hussain, J.; Hasan, A. Sodium alginate-f-GO composite hydrogels for tissue regeneration and antitumor applications. Int. J. Biol. Macromol. 2022, 208, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Zamri, M.F.M.A.; Bahru, R.; Amin, R.; Khan, M.U.A.; Abd Razak, S.I.; Hassan, S.A.; Kadir, M.R.A.; Nayan, N.H.M. Waste to health: A review of waste derived materials for tissue engineering. J. Clean. Prod. 2021, 290, 125792. [Google Scholar] [CrossRef]
- Huang, J.P.; Mojib, N.; Goli, R.R.; Watkins, S.; Waites, K.B.; Ravindra, R.; Andersen, D.T.; Bej, A.K. Antimicrobial activity of PVP from an Antarctic bacterium, Janthinobacterium sp. Ant5-2, on multi-drug and methicillin resistant Staphylococcus aureus. Nat. Prod. Bioprospect. 2012, 2, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Aslam Khan, M.U.; Mehboob, H.; Abd Razak, S.I.; Yahya, M.Y.; Mohd Yusof, A.H.; Ramlee, M.H.; Sahaya Anand, T.J.; Hassan, R.; Aziz, A.; Amin, R. Development of polymeric nanocomposite (xyloglucan-co-methacrylic acid/hydroxyapatite/sio2) scaffold for bone tissue engineering applications—in-vitro antibacterial, cytotoxicity and cell culture evaluation. Polymers 2020, 12, 1238. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Khan, M.U.; Akbar, J.; Shad, M.A.; Masih, R.; Chaudhary, M.T. Isoconversional thermal and pyrolytic GC–MS analysis of street samples of hashish. J. Anal. Appl. Pyrolysis 2016, 122, 175–182. [Google Scholar] [CrossRef]
- Mirzaie, Z.; Reisi-Vanani, A.; Barati, M. Polyvinyl alcohol-sodium alginate blend, composited with 3D-graphene oxide as a controlled release system for curcumin. J. Drug Deliv. Sci. Technol. 2019, 50, 380–387. [Google Scholar] [CrossRef]










| Drug Release at Different pH-Levels | Models | Intercept/Standard Error | Slop/Standard Error | Regression Coefficient (R2) |
|---|---|---|---|---|
| Drug release kinetics at pH 6.4 | Zero order | −0.52979/0.37126 | 0.48568/0.01755 | 0.98837 |
| First order | 0.16701/0.09621 | 0.03517/0.00455 | 0.86721 | |
| Higuchi | −14.19939/6.18472 | 13.67722/1.49206 | 0.89251 | |
| Korsmeyer-Peppas | 0.94986/0.17899 | 0.78764/0.15055 | 0.74554 | |
| Hixson | −0.7623/0.2354 | 33.8974/0.47476 | 0.99054 | |
| Bakers-Lonsdale | −18,183.23/10,589.06 | 9974.14/2554.60 | 0.58753 | |
| Drug release kinetics at pH 7.4 | Zero order | 0.8095/0.21262 | 0.2173/0.01005 | 098106 |
| First order | 0.14378/0.07845 | 0.02573/0.00371 | 0.83965 | |
| Higuchi | −11.10565/4.33 | 17.22369/1.04596 | 0.96431 | |
| Korsmeyer-Peppas | 1.4071/0.15032 | 0.56002/0.12644 | 0.67412 | |
| Hixson | 31.14956/0.84 | −0.92297/0.04151 | 0.98014 | |
| Bakers-Lonsdale | −80,632.11/21,021.06 | 32,004.02/4835.30 | 0.82628 | |
| Drug release kinetics at pH 8.4 | Zero order | −2.2668/0.65 | 0.61835/0.03 | 0.9782 |
| First order | 0.04723/0.08 | 0.04155/0.003 | 0.93277 | |
| Higuchi | −12.57362/5.93 | 9.85459/1.43 | 0.82256 | |
| Korsmeyer-Peppas | 0.59247/0.19 | 0.91234/0.16 | 0.76697 | |
| Hixson | 34.79252/0.59 | −0.56507/0.03 | 0.97366 | |
| Bakers-Lonsdale | −15,727.59/5918.95 | 5546.23/1361.49 | 0.63407 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Arjan, W.S.; Khan, M.U.A.; Almutairi, H.H.; Alharbi, S.M.; Razak, S.I.A. pH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics. Polymers 2022, 14, 1949. https://doi.org/10.3390/polym14101949
Al-Arjan WS, Khan MUA, Almutairi HH, Alharbi SM, Razak SIA. pH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics. Polymers. 2022; 14(10):1949. https://doi.org/10.3390/polym14101949
Chicago/Turabian StyleAl-Arjan, Wafa Shamsan, Muhammad Umar Aslam Khan, Hayfa Habes Almutairi, Shadia Mohammed Alharbi, and Saiful Izwan Abd Razak. 2022. "pH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics" Polymers 14, no. 10: 1949. https://doi.org/10.3390/polym14101949