In Vivo Study of Nasal Bone Reconstruction with Collagen, Elastin and Chitosan Membranes in Abstainer and Alcoholic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
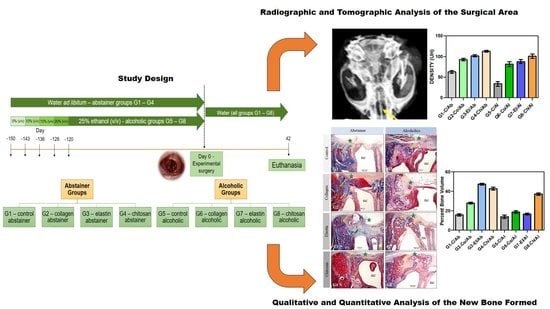
2.2. Induction of Alcoholism and Experimental Groups
2.3. Fabrication of the Biomaterials
2.4. Surgical Procedure
2.5. Euthanasia
2.6. Radiographic and Tomographic Analysis of the Surgical Area
2.7. Histological and Histomorphometric Analysis of the Surgical Area
2.8. Statistical Analysis
3. Results
3.1. Radiographic and Tomographic Analysis
3.2. Histological Analysis of the Surgical Area
3.3. Histomorphometric and Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Baraúna Magno, M.; de França Leite, K.L.; Melo Pithon, M.; Maia, L.C. Are traumatic dental injuries greater in alcohol or illicit drugs consumers? A systematic review and meta-analysis. Drug Alcohol Depend. 2019, 197, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Hirvikangas, R.; Bertell, J.; Marttila, E.; Löfgren, M.; Snäll, J.; Uittamo, J. Patient injury-related alcohol use—Underestimated in patients with facial fractures? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 236–240. [Google Scholar] [CrossRef]
- Lee, K.; Olsen, J.; Sun, J.; Chandu, A. Alcohol-involved maxillofacial fractures. Aust. Dent. J. 2017, 62, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Mast, G.; Ehrenfeld, M.; Cornelius, C.P.; Litschel, R.; Tasman, A.J. Maxillofacial Fractures: Midface and Internal Orbit-Part I: Classification and Assessment. Facial Plast. Surg. 2015, 31, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Mast, G.; Ehrenfeld, M.; Cornelius, C.P.; Tasman, A.J.; Litschel, R. Maxillofacial Fractures: Midface and Internal Orbit-Part II: Principles and Surgical Treatment. Facial Plast. Surg. 2015, 31, 357–367. [Google Scholar] [CrossRef] [PubMed]
- González-Reimers, E.; Quintero-Platt, G.; Rodríguez-Rodríguez, E.; Martínez-Riera, A.; Alvisa-Negrín, J.; Santolaria-Fernández, F. Bone changes in alcoholic liver disease. World J. Hepatol. 2015, 7, 1258–1264. [Google Scholar] [CrossRef]
- López-Larramona, G.; Lucendo, A.J.; González-Delgado, L. Alcoholic liver disease and changes in bone mineral density. Rev. Española Enferm. Dig. 2013, 105, 609–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.K.; Callaci, J.J.; Lauing, K.L.; Otis, J.S.; Radek, K.A.; Jones, M.K.; Kovacs, E.J. Alcohol Exposure and Mechanisms of Tissue Injury and Repair. Alcohol. Clin. Exp. Res. 2011, 35, 392–399. [Google Scholar] [CrossRef]
- Pomini, K.T.; Cestari, T.M.; Santos German, Í.J.; de Oliveira Rosso, M.P.; de Oliveira Gonçalves, J.B.; Buchaim, D.V.; Pereira, M.; Andreo, J.C.; Rosa, G.M.; Della Coletta, B.B.; et al. Influence of experimental alcoholism on the repair process of bone defects filled with beta-tricalcium phosphate. Drug Alcohol Depend. 2019, 197, 315–325. [Google Scholar] [CrossRef]
- German, I.J.S.; Pomini, K.T.; Bighetti, A.C.C.; Andreo, J.C.; Reis, C.H.B.; Shinohara, A.L.; Rosa, G.M.; de Bortoli Teixeira, D.; de Oliveira Rosso, M.P.; Buchaim, D.V.; et al. Evaluation of the use of an inorganic bone matrix in the repair of bone defects in rats submitted to experimental alcoholism. Materials 2020, 13, 695. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar] [CrossRef]
- Della Coletta, B.B.; Jacob, T.B.; Moreira, L.A.d.C.; Pomini, K.T.; Buchaim, D.V.; Eleutério, R.G.; Pereira, E.d.S.B.M.; Roque, D.D.; Rosso, M.P.O.; Shindo, J.V.T.C.; et al. Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules 2021, 26, 847. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46, 103–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Fernández-Bodereau, E.; Dedossi, G.; Asencio, V.; Fernández-Domínguez, M.; Gehrke, S.; Aragoneses, J.; Calvo-Guirado, J. Comparison of different bone filling materials and resorbable membranes by means of micro-tomography. A preliminary study in Rabbits. Materials 2019, 12, 1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettian, M.S.; De Guzzi Plepis, A.M.; Da Conceição Amaro Martins, V.; Dos Santos, G.R.; Lopes Pinto, C.A.; Galdeano, E.A.; Alves Calegari, A.R.; De Moraes, C.A.; Da Cunha, M.R. Use of an anionic collagen matrix made from bovine intestinal serosa for in vivo repair of cranial defects. PLoS ONE 2018, 13, e0197806. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.L.; Wan, L.; Wang, S.K. The study of the feasibility of segmental bone defect repair with tissue- engineered bone membrane: A qualitative observation. Strateg. Trauma Limb Reconstr. 2008, 3, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, R.L.; Goissis, G.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Buchaim, D.V.; Rodrigues, A.C. Biocompatibility of anionic collagen matrices and its influence on the orientation of cellular growth. Braz. Dent. Sci. 2007, 10, 12–20. [Google Scholar] [CrossRef]
- Yeo, G.C.; Aghaei-Ghareh-Bolagh, B.; Brackenreg, E.P.; Hiob, M.A.; Lee, P.; Weiss, A.S. Fabricated Elastin. Adv. Healthc. Mater. 2015, 4, 2530–2556, Correction in 2018, 7, 3–5, doi:10.1002/adhm.201801342. [Google Scholar] [CrossRef] [Green Version]
- Daamen, W.F.; Veerkamp, J.H.; van Hest, J.C.M.; van Kuppevelt, T.H. Elastin as a biomaterial for tissue engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef]
- De Aragão Tavares, E.; De Medeiros, W.M.T.Q.; De Assis Pontes, T.P.; Barbosa, M.M.; De Araújo, A.A.; De Araújo, R.F.; Figueiredo, J.G.; Leitão, R.C.; Da Silva Martins, C.; Da Silva, F.O.N.; et al. Chitosan membrane modified with a new zinc(II)-vanillin complex improves skin wound healing in diabetic rats. Front. Pharmacol. 2019, 9, 1511. [Google Scholar] [CrossRef]
- Çelebi, B.; Cloutier, M.; Balloni, R.; Mantovani, D.; Bandiera, A. Human Elastin-Based Recombinant Biopolymers Improve Mesenchymal Stem Cell Differentiation. Macromol. Biosci. 2012, 12, 1546–1554. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the Arrive Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- de Moraes, R.; de Guzzi Plepis, A.M.; da Conceição Amaro Martins, V.; Duarte, M.A.H.; Alcalde, M.P.; Buchaim, R.L.; Pomini, K.T.; Machado, E.G.; de Azevedo E Sousa Munhoz, M.; Cunha, F.B.; et al. Suitability of the use of an elastin matrix combined with bone morphogenetic protein for the repair of cranial defects. Am. J. Transl. Res. 2019, 11, 5261–5271. [Google Scholar]
- De Azevedo e Sousa Munhoz, M.; Pomini, K.T.; de Guzzi Plepis, A.M.; da Conceição Amaro Martins, V.; Machado, E.G.; de Moraes, R.; Cunha, F.B.; Santos, A.R.; Cardoso, G.B.C.; Duarte, M.A.H.; et al. Elastin-derived scaffolding associated or not with bone morphogenetic protein (BMP) or hydroxyapatite (HA) in the repair process of metaphyseal bone defects. PLoS ONE 2020, 15, e0231112. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, D.A.; de Guzzi Plepis, A.M.; da Conceição Amaro Martins, V.; Cardoso, G.B.C.; Santos, A.R.; Iatecola, A.; Andrade, T.N.; Monteiro, F.M.; Calegari, A.R.A.; Chacon, E.L.; et al. Effects of the combination of low-level laser therapy and anionic polymer membranes on bone repair. Lasers Med. Sci. 2020, 35, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Massimino, L.C.; da Conceição Amaro Martins, V.; Vulcani, V.A.S.; de Oliveira, É.L.; Andreeta, M.B.; Bonagamba, T.J.; Klingbeil, M.F.G.; Mathor, M.B.; de Guzzi Plepis, A.M. Use of collagen and auricular cartilage in bioengineering: Scaffolds for tissue regeneration. Cell Tissue Bank. 2020. [Google Scholar] [CrossRef]
- Horn, M.M.; Martins, V.C.A.; de Guzzi Plepis, A.M. Interaction of anionic collagen with chitosan: Effect on thermal and morphological characteristics. Carbohydr. Polym. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Cunha, F.B.; Pomini, K.T.; Plepis, A.M.d.G.; Martins, V.d.C.A.; Machado, E.G.; de Moraes, R.; Munhoz, M.d.A.E.S.; Machado, M.V.R.; Duarte, M.A.H.; Alcalde, M.P.; et al. In Vivo Biological Behavior of Polymer Scaffolds of Natural Origin in the Bone Repair Process. Molecules 2021, 26, 1598. [Google Scholar] [CrossRef]
- Silva, I.M.D.C.C.; De Freitas, D.Q.; Ambrosano, G.M.B.; Bóscolo, F.N.; Almeida, S.M. Bone density: Comparative evaluation of hounsfield units in multislice and cone-beam computed tomography. Braz. Oral Res. 2012, 26, 550–556. [Google Scholar] [CrossRef]
- Melo, L.G.N.; Nagata, M.J.H.; Bosco, A.F.; Ribeiro, L.L.G.; Leite, C.M. Bone healing in surgically created defects treated with either bioactive glass particles, a calcium sulfate barrier, or a combination of both materials: A histological and histometric study in rat tibias. Clin. Oral Implant. Res. 2005, 16, 683–691. [Google Scholar] [CrossRef]
- Messora, M.R.; Nagata, M.J.H.; Mariano, R.C.; Dornelles, R.C.M.; Bomfim, S.R.M.; Fucini, S.E.; Garcia, V.G.; Bosco, A.F. Bone healing in critical-size defects treated with platelet-rich plasma: A histologic and histometric study in rat calvaria. J. Periodontal Res. 2008, 43, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Fontes Martins, L.C.; Sousa Campos de Oliveira, A.L.; Aloise, A.C.; Scavone de Macedo, L.G.; Teixeira, M.L.; Moy, P.K.; Pelegrine, A.A. Bone marrow aspirate concentrate and platelet-rich fibrin in fresh extraction sockets: A histomorphometric and immunohistochemical study in humans. J. Cranio Maxillofac. Surg. 2021, 49, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Iatecola, A.; Barraviera, B.; Junior, R.S.F.; dos Santos, G.R.; Neves, J.I.; da Cunha, M.R. Use of a new fibrin sealant and laser irradiation in the repair of skull defects in rats. Braz. Dent. J. 2013, 24, 456–461. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Gonçalves, J.B.; Buchaim, D.V.; de Souza Bueno, C.R.; Pomini, K.T.; Barraviera, B.; Júnior, R.S.F.; Andreo, J.C.; de Castro Rodrigues, A.; Cestari, T.M.; Buchaim, R.L. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J. Photochem. Photobiol. B Biol. 2016, 162, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, D.V.; Cassaro, C.V.; Shindo, J.V.T.C.; Coletta, B.B.D.; Pomini, K.T.; Rosso, M.P.O.; Campos, L.M.G.; Ferreira Jr, R.S.; Barraviera, B.; Buchaim, R.L. Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties—A systematic review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190038. [Google Scholar] [CrossRef]
- Rosso, M.P.d.O.; Buchaim, D.V.; Pomini, K.T.; Coletta, B.B.D.; Reis, C.H.B.; Pilon, J.P.G.; Júnior, G.D.; Buchaim, R.L. Photobiomodulation therapy (PBMT) applied in bone reconstructive surgery using bovine bone grafts: A systematic review. Materials 2019, 12, 4051. [Google Scholar] [CrossRef] [Green Version]
- Pomini, K.T.; Buchaim, D.V.; Andreo, J.C.; Rosso, M.P.d.O.; Della Coletta, B.B.; German, Í.J.S.; Biguetti, A.C.C.; Shinohara, A.L.; Rosa Júnior, G.M.; Shindo, J.V.T.C.; et al. Fibrin sealant derived from human plasma as a scaffold for bone grafts associated with photobiomodulation therapy. Int. J. Mol. Sci. 2019, 20, 1761. [Google Scholar] [CrossRef] [Green Version]
- Rosso, M.P.O.; Oyadomari, A.T.; Pomini, K.T.; Della Coletta, B.B.; Shindo, J.V.T.C.; Ferreira, R.S., Jr.; Barraviera, B.; Cassaro, C.V.; Buchaim, D.V.; Teixeira, D.D.B.; et al. Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones. Biomolecules 2020, 10, 383. [Google Scholar] [CrossRef] [Green Version]
- Maciel, J.; Momesso, G.; Ramalho-Ferreira, G.; Consolaro, R.; Perri de Carvalho, P.; Faverani, L.; Farnezi Bassi, A.; Gosain, A.; Song, L.; Yu, P.; et al. Bone Healing Evaluation in Critical-Size Defects Treated With Xenogenous Bone Plus Porcine Collagen. Implant Dent. 2017, 26, 296–302. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Dos Santos Bueno, P.C.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Zilio, M.G.; Salatin, J.A.; Kawano, N.; Furlanette, G.; Buchaim, R.L. Action of a deproteinized xenogenic biomaterial in the process of bone repair in rats submitted to inhalation of cigarette smoke. Acta Cir. Bras. 2018, 33, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]
- Bardsley, K.; Kwarciak, A.; Freeman, C.; Brook, I.; Hatton, P.; Crawford, A. Repair of bone defects In Vivo using tissue engineered hypertrophic cartilage grafts produced from nasal chondrocytes. Biomaterials 2017, 112, 313–323. [Google Scholar] [CrossRef]
- Jeynes, K.D.; Gibson, E.L. The importance of nutrition in aiding recovery from substance use disorders: A review. Drug Alcohol Depend. 2017, 179, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkawi, I.; Zmerly, H. Osteoporosis: Current Concepts. Osteoporosis 2018, 6, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; He, X.; Jin, D.; Wu, S.; Wang, H.; Yin, M.; Aldalbahi, A.; El-Newehy, M.; Mo, X.; Wu, J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020, 108, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Veríssimo, D.M.; Leitão, R.F.; Figueiró, S.D.; Góes, J.C.; Lima, V.; Silveira, C.O.; Brito, G.A. Guided bone regeneration produced by new mineralized and reticulated collagen membranes in critical-sized rat calvarial defects. Exp. Biol. Med. 2015, 240, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.A.; Benítez-García, J.A.; Osorio, R. Differential biodegradation kinetics of collagen membranes for bone regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef]
- Moses, O.; Nemcovsky, C.E.; Tal, H.; Zohar, R. Tetracycline Modulates Collagen Membrane Degradation In Vitro. J. Periodontol. 2001, 72, 1588–1593. [Google Scholar] [CrossRef]
- Zohar, R.; Nemcovsky, C.E.; Kebudi, E.; Artzi, Z.; Tal, H.; Moses, O. Tetracycline Impregnation Delays Collagen Membrane Degradation In Vivo. J. Periodontol. 2004, 75, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, B.; Bierdeman, P.C.; Janorkar, A.V. Composition of elastin like polypeptide–collagen composite scaffold influences in vitro osteogenic activity of human adipose derived stem cells. Dent. Mater. 2016, 32, 1270–1280. [Google Scholar] [CrossRef] [Green Version]
- Góes, J.C.; Figueiró, S.D.; Oliveira, A.M.; Macedo, A.A.M.; Silva, C.C.; Ricardo, N.M.P.S.; Sombra, A.S.B. Apatite coating on anionic and native collagen films by an alternate soaking process. Acta Biomater. 2007, 3, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.J. Potential use of collagen and elastin degradation markers for monitoring liver fibrosis in schistosomiasis. Acta Trop. 2000, 77, 97–99. [Google Scholar] [CrossRef]
- Partridge, C.R.; Sampsont, H.W.; Forough, R. Long-term alcohol consumption increases matrix metalloproteinase-2 activity in rat aorta. Life Sci. 1999, 65, 1395–1402. [Google Scholar] [CrossRef]
- Costa, A.; Ceresa, D.; De Palma, A.; Rossi, R.; Turturo, S.; Santamaria, S.; Balbi, C.; Villa, F.; Reverberi, D.; Cortese, K.; et al. Comprehensive profiling of secretome formulations from fetal-and perinatal human amniotic fluid stem cells. Int. J. Mol. Sci. 2021, 22, 3713. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Katayama, Y. Biomaterial-assisted regenerative medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef] [Green Version]
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Esposti, M.D.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L.; et al. PLA-based mineral-doped scaffolds seeded with human periapical cyst-derived MSCs: A promising tool for regenerative healing in dentistry. Materials 2019, 12, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, H.J.; Lee, G.Y.; Yang, S.K.; Kim, D.S.; Yun, K.J.; Kim, E.C.; Kim, H.M.; Chae, S.W.; Kim, H.R. Effect of high molecular weight water-soluble chitosan on the trabecular bone and thickness in ovariectomized rats. Immunopharmacol. Immunotoxicol. 2007, 29, 439–449. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, L.L.; Ricci, V.P.; Prado, D.G.; Apolinario, R.C.; De Oliveira Vercik, L.C.; Da Silva Rigo, E.C.; Dos Santos Fernandes, M.C.; Mariano, N.A. Titanium coating with hydroxyapatite and chitosan doped with silver nitrate. Mater. Res. 2017, 20, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Chakkalakal, D.A. Alcohol-induced bone loss and deficient bone repair. Alcohol. Clin. Exp. Res. 2005, 29, 2077–2090. [Google Scholar] [CrossRef]
- de Almeida, A.L.P.F.; Medeiros, I.L.; Cunha, M.J.S.; Sbrana, M.C.; de Oliveira, P.G.F.P.; Esper, L.A. The effect of low-level laser on bone healing in critical size defects treated with or without autogenous bone graft: An experimental study in rat calvaria. Clin. Oral Implant. Res. 2014, 25, 1131–1136. [Google Scholar] [CrossRef]
- Escudero, J.S.B.; Perez, M.G.B.; de Oliveira Rosso, M.P.; Buchaim, D.V.; Pomini, K.T.; Campos, L.M.G.; Audi, M.; Buchaim, R.L. Photobiomodulation therapy (PBMT) in bone repair: A systematic review. Injury 2019, 50, 1853–1867. [Google Scholar] [CrossRef]





| Groups | Mean ± SD |
|---|---|
| G1—control/abstainer (C/Ab) | 15.78 ± 1.19 fg |
| G2—collagen/abstainer (Co/Ab) | 27.81 ± 0.91 d |
| G3—elastin/abstainer (El/Ab) | 47.29 ± 0.97 a |
| G4—chitosan/abstainer (Ch/Ab) | 42.69 ± 1.52 b |
| G5—control/alcoholic (C/Al) | 13.81 ± 1.60 g |
| G6—collagen/alcoholic (Co/Al) | 18.59 ± 1.37 e |
| G7—elastin/alcoholic (El/Al) | 16.54 ± 0.89 ef |
| G8—chitosan/alcoholic (Ch/Al) | 37.06 ± 1.17 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandini, F.E.; Kubo, F.M.M.; Plepis, A.M.d.G.; Martins, V.d.C.A.; da Cunha, M.R.; Silva, V.R.; Hirota, V.B.; Lopes, E.; Menezes, M.A.; Pelegrine, A.A.; et al. In Vivo Study of Nasal Bone Reconstruction with Collagen, Elastin and Chitosan Membranes in Abstainer and Alcoholic Rats. Polymers 2022, 14, 188. https://doi.org/10.3390/polym14010188
Pandini FE, Kubo FMM, Plepis AMdG, Martins VdCA, da Cunha MR, Silva VR, Hirota VB, Lopes E, Menezes MA, Pelegrine AA, et al. In Vivo Study of Nasal Bone Reconstruction with Collagen, Elastin and Chitosan Membranes in Abstainer and Alcoholic Rats. Polymers. 2022; 14(1):188. https://doi.org/10.3390/polym14010188
Chicago/Turabian StylePandini, Fabricio Egidio, Fabíola Mayumi Miyauchi Kubo, Ana Maria de Guzzi Plepis, Virginia da Conceição Amaro Martins, Marcelo Rodrigues da Cunha, Vinicius Rodrigues Silva, Vinicius Barroso Hirota, Everton Lopes, Marcos Antonio Menezes, André Antonio Pelegrine, and et al. 2022. "In Vivo Study of Nasal Bone Reconstruction with Collagen, Elastin and Chitosan Membranes in Abstainer and Alcoholic Rats" Polymers 14, no. 1: 188. https://doi.org/10.3390/polym14010188