Hydrophobically-Modified PEG Hydrogels with Controllable Hydrophilic/Hydrophobic Balance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
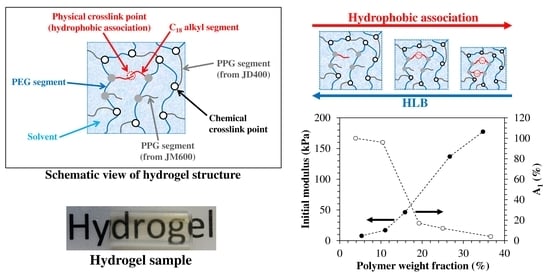
2.2. Synthesis of HM Hydrogels
2.3. FTIR Analysis
2.4. TGA Analysis
2.5. Swelling Tests
2.6. LF-NMR Experiments
2.7. Uniaxial Tensile Tests
3. Results and Discussion
3.1. Hydrogel Preparation
3.2. LF-NMR Analysis
3.3. Room Temperature Swelling Behavior
3.4. Thermo- and pH-Sensitivity in Aqueous Media
3.5. Tensile Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Buengera, D.; Topuza, F.; Groll, J. Hydrogels in sensing applications. Progr. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Dave, A.M.; Vaishnav, U.H.; Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, T.M. Hydrogels as controlled release devices in agriculture. Des. Monomers Polym. 2002, 5, 39–65. [Google Scholar] [CrossRef]
- Jing, G.; Wang, L.; Yu, H.; Amer, W.A.; Zhang, L. Recent progress on study of hybrid hydrogels for water treatment. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 86–94. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Rogovina, L.Z.; Vasil’ev, V.G.; Braudo, E.E. Definition of the concept of polymer gel. Polym. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Hirai, Y.; Terashima, T.; Takenaka, M.; Sawamoto, M. Precision self-assembly of amphiphilic random copolymers into uniform and self-sorting nanocompartments in water. Macromolecules 2016, 49, 5084–5091. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended assemblies of amphiphilic random and block copolymers for tunable encapsulation and release of hydrophobic guest. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Missirlis, D.; Tirelli, N.; Hubbell, J.A. Amphiphilic hydrogel nanoparticles. Preparation, characterization, and preliminary assessment as new colloidal drug carriers. Langmuir 2005, 21, 2605–2613. [Google Scholar] [CrossRef]
- Kafouris, D.; Gradzielski, M.; Patrickios, C.S. Semisegmented amphiphilic polymer conetworks: Synthesis and characterization. Macromolecules 2009, 42, 2972–2980. [Google Scholar] [CrossRef]
- Truong, V.; Blakey, I.; Whittaker, A.K. Hydrophilic and amphiphilic polyethylene glycol-based hydrogels with tunable degradability prepared by “click” chemistry. Biomacromolecules 2012, 13, 4012–4021. [Google Scholar] [CrossRef]
- Wang, S.; Attah, R.; Li, J.; Chen, Y.; Chen, R. A pH-responsive amphiphilic hydrogel based on pseudopeptides and poly(ethylene glycol) for oral delivery of hydrophobic drugs. ACS Biomater. Sci. Eng. 2018, 4, 4236–4243. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; O’Connor, A.J.; Qiao, G.G.H.; Ladewig, K. Hydrogels with smart systems for delivery of hydrophobic drugs. Expert Opin. Drug Deliv. 2017, 14, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and self-healing hydrogels formed via hydrophobic interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Xu, K.; An, H.; Lu, C.; Tan, Y.; Li, P.; Wang, P. Facile fabrication method of hydrophobic-associating cross-linking hydrogel with outstanding mechanical performance and self-healing property in the absence of surfactants. Polymer 2013, 54, 5665–5672. [Google Scholar] [CrossRef]
- Abdurrahmanoglu, S.; Can, V.; Okay, O. Design of high-toughness polyacrylamide hydrogels by hydrophobic modification. Polymer 2009, 50, 5449–5455. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, C.; Liu, X.; Zhang, G.; Yang, M.; Liu, F. Construction and properties of hydrophobic association hydrogels with high mechanical strength and reforming capability. Macromol. Mater. Eng. 2009, 294, 815–820. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Yu, J.; Gao, G.; Liu, F. Network structure and mechanical properties of hydrophobic association hydrogels: Surfactant effect I. J. Appl. Polym. Sci. 2015, 132, 41222. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Li, Z.; Gao, G.; Liu, F. Temperature-responsive properties of poly(acrylic acid-co-acrylamide) hydrophobic association hydrogels with high mechanical strength. Macromolecules 2010, 43, 10645–10651. [Google Scholar] [CrossRef]
- Gholap, S.G.; Jog, J.P.; Badiger, M.V. Synthesis and characterization of hydrophobically modified poly(vinyl alcohol) hydrogel membrane. Polymer 2004, 45, 5863–5873. [Google Scholar] [CrossRef]
- Miquelard-Garnier, G.; Demoures, S.; Creton, C.; Hourdet, D. Synthesis and rheological behavior of new hydrophobically modified hydrogels with tunable properties. Macromolecules 2006, 39, 8128–8139. [Google Scholar] [CrossRef]
- Erdodi, G.; Ivan, B. Novel amphiphilic conetworks composed of telechelic poly(ethylene oxide) and three-arm star polyisobutylene. Chem. Mater. 2004, 16, 959–962. [Google Scholar] [CrossRef]
- Peppas, N.A.; Keys, K.B.; Torres-Lugo, M.; Lowman, A.M. Poly(ethylene glycol)-containing hydrogels in drug delivery. J. Control. Release 1999, 62, 81–87. [Google Scholar] [CrossRef]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M. Polymerization of ethylene oxide, propylene oxide, and other alkylene oxides: Synthesis, novel polymer architectures, and bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.K. Liposomes for enhanced bioavailability of water-insoluble drugs: In vivo evidence and recent approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [Green Version]
- Vhora, I.; Lalani, R.; Bhatt, P.; Patil, S.; Patel, H.; Patel, V.; Misra, A. Colloidally stable small unilamellar stearyl amine lipoplexes for effective BMP-9 gene delivery to stem cells for osteogenic differentiation. AAPS PharmSciTech 2018, 19, 3550–3560. [Google Scholar] [CrossRef]
- ASTM D1652, Test Method for Epoxy Content of Epoxy Resins. 2004. Available online: http://materialstandard.com/wp-content/uploads/2020/01/D1652-11e1.pdf (accessed on 1 January 2004).
- Meiboom, S.; Gill, D. Modified spin-echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Brownstein, K.R.; Tarr, C.E. Importance of classical diffusion in NMR studies of water in biological cells. Phys. Rev. A 1979, 19, 2446–2453. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis, 1st ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1966. [Google Scholar]
- Tanaka, Y.; Bauer, R.S. Curing Reactions. In Epoxy Resins: Chemistry and Technology, 2nd ed.; May, C.A., Ed.; Marcel Dekker: New York, NY, USA, 1988; pp. 285–463. [Google Scholar]
- Pascault, J.P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers; Marcel Dekker: New York, NY, USA, 2002; pp. 83–97. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; pp. 576–584. [Google Scholar]
- Van Krevelen, D.W.; Nijenhuis, K. Properties of Polymers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; p. 765. [Google Scholar]
- Chui, M.M.; Phillips, R.J.; McCarthy, M.J. Measurement of the porous microstructure of hydrogels by nuclear magnetic resonance. J. Colloid Interface Sci. 1995, 174, 336–344. [Google Scholar] [CrossRef]
- Abrami, M.; Chiarappa, G.; Farra, R.; Grassi, G.; Marizza, P.; Grassi, M. Use of low-field NMR for the characterization of gels and biological tissues. ADMET DMPK 2018, 6, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Tam, K.C. Isothermal titration calorimetric studies on the temperature dependence of binding interactions between poly(propylene glycol)s and sodium dodecyl sulfate. Langmuir 2004, 20, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Rad, E.R.; Vahabi, H.; Formela, K.; Saeb, M.R.; Thomas, S. Injectable poloxamer/graphene oxide hydrogels with well-controlled mechanical and rheological properties. Polym. Adv. Technol. 2019, 30, 2250–2260. [Google Scholar]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Armstrong, J.; Chowdhry, B.; Mitchell, J.; Beezer, A.; Leharne, S. Effect of cosolvents and cosolutes upon aggregation transitions in aqueous solutions of the poloxamer F87 (poloxamer P237): A high sensitivity differential scanning calorimetry study. J. Phys. Chem. 1996, 100, 1738–1745. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Peppas, N.A. Equilibrium swelling behavior of pH-sensitive hydrogels. Chem. Eng. Sci. 1991, 46, 715–722. [Google Scholar] [CrossRef]
- Bueche, F.; Halpin, J.C. Molecular theory for the tensile strength of gum elastomers. J. Appl. Phys. 1964, 35, 36–41. [Google Scholar] [CrossRef]
- Shibayama, M. Small-angle neutron scattering on polymer gels: Phase behavior, inhomogeneities and deformation mechanisms. Polym. J. 2011, 43, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Sun, T.L.; Nakajima, T.; Kurokawa, T.; Zhao, Y.; Sato, K.; Ihsan, A.B.; Li, X.; Guo, H.; Gong, J.P. Oppositely charged polyelectrolytes form tough, self-healing, and rebuildable hydrogels. Adv. Mater. 2015, 27, 2722–2727. [Google Scholar] [CrossRef]
- Hu, X.; Vatankhah-Varnoosfaderani, M.; Zhou, J.; Li, Q.; Sheiko, S.S. Weak hydrogen bonding enables hard, strong, tough, and elastic hydrogels. Adv. Mater. 2015, 27, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Weiss, R.A. Viscoelastic and mechanical behavior of hydrophobically modified hydrogels. Macromolecules 2011, 44, 9390–9398. [Google Scholar] [CrossRef]
- Brown, H.R. A model of the fracture of double network gels. Macromolecules 2007, 40, 3815–3818. [Google Scholar] [CrossRef]
- Rubinstein, M.; Dobrynin, A.V. Associations leading to formation of reversible networks and gels. Curr. Opin. Colloid Interface Sci. 1999, 4, 83–87. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]












| Code | q | fJD400 | fODA | ODA (wt%) | JM600 (wt%) | JD400 (wt%) | PEGDE (wt%) |
|---|---|---|---|---|---|---|---|
| HM-40-00 | 0.80 | 0.40 | 0.00 | 0 | 42.4 | 10.5 | 47.1 |
| HM-40-25 | 0.80 | 0.40 | 0.25 | 4.8 | 33.8 | 11.2 | 50.2 |
| HM-40-50 | 0.80 | 0.40 | 0.50 | 10.3 | 24.1 | 12.0 | 53.6 |
| HM-40-75 | 0.80 | 0.40 | 0.75 | 16.5 | 13.0 | 12.9 | 57.6 |
| HM-40-100 | 0.80 | 0.40 | 1.00 | 23.9 | 0 | 13.9 | 62.2 |
| HM-48-75 | 0.80 | 0.48 | 0.75 | 14.6 | 11.3 | 15.7 | 58.4 |
| HM-56-75 | 0.80 | 0.56 | 0.75 | 12.5 | 9.8 | 18.5 | 59.2 |
| Code | Initial Weight Loss (%) | Tpeak1 a (°C) | WL1 b (%) | Tpeak2 a (°C) | WL2 b (%) | Residue at 700 °C (%) |
|---|---|---|---|---|---|---|
| HM-40-00 | 3.1 | 301 | 46.7 | 355 | 48.0 | 1.8 |
| HM-40-25 | 3.5 | 311 | 44.6 | 352 | 49.2 | 2.5 |
| HM-40-50 | 2.9 | 307 | 40.7 | 353 | 53.6 | 3.5 |
| HM-40-75 | 2.2 | 312 | 40.3 | 354 | 55.5 | 1.6 |
| HM-40-100 | 2.4 | 318 | 37.8 | 353 | 57.0 | 2.0 |
| HM-48-75 | 3.6 | 308 | 37.9 | 358 | 56.6 | 1.0 |
| HM-56-75 | 2.8 | 309 | 36.6 | 358 | 58.4 | 1.7 |
| Code | fODA | T2m (ms) | T21 (ms) | A1 (%) | T22 (ms) | A2 (%) |
|---|---|---|---|---|---|---|
| HM-40-00 | 0.00 | 2332 | 2332 | 100 | - | 0 |
| HM-40-25 | 0.25 | 1415 | 1460 | 96 | 191 | 4 |
| HM-40-50 | 0.50 | 861 | 1607 | 17 | 709 | 83 |
| HM-40-75 | 0.75 | 571 | 1931 | 12 | 384 | 88 |
| HM-40-100 | 1.00 | 204 | 695 | 4 | 186 | 96 |
| Code | q | fJD400 | fODA | wp (%) | Ein a (kPa) | εb b (%) | σb c (kPa) |
|---|---|---|---|---|---|---|---|
| HM-40-00 | 0.80 | 0.40 | 0.00 | 5.3 ± 0.2 | 8 ± 1 | 26 ± 6 | 1.6 ± 0.5 |
| HM-40-25 | 0.80 | 0.40 | 0.25 | 11.1 ± 0.2 | 17 ± 2 | 60 ± 15 | 8 ± 2 |
| HM-40-50 | 0.80 | 0.40 | 0.50 | 16 ± 1 | 45 ± 5 | 100 ± 30 | 26 ± 6 |
| HM-40-75 | 0.80 | 0.40 | 0.75 | 26.7 ± 0.1 | 140 ± 10 | 260 ± 30 | 120 ± 20 |
| HM-40-100 | 0.80 | 0.40 | 1.00 | 34.8 ± 0.1 | 180 ± 20 | 450 ± 20 | 160 ± 15 |
| HM-48-75 | 0.80 | 0.48 | 0.75 | 28.8 ± 0.2 | 260 ± 20 | 90 ± 10 | 123 ± 7 |
| HM-56-75 | 0.80 | 0.56 | 0.75 | 31.1 ± 0.1 | 540 ± 30 | 35 ± 5 | 145 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bignotti, F.; Baldi, F.; Grassi, M.; Abrami, M.; Spagnoli, G. Hydrophobically-Modified PEG Hydrogels with Controllable Hydrophilic/Hydrophobic Balance. Polymers 2021, 13, 1489. https://doi.org/10.3390/polym13091489
Bignotti F, Baldi F, Grassi M, Abrami M, Spagnoli G. Hydrophobically-Modified PEG Hydrogels with Controllable Hydrophilic/Hydrophobic Balance. Polymers. 2021; 13(9):1489. https://doi.org/10.3390/polym13091489
Chicago/Turabian StyleBignotti, Fabio, Francesco Baldi, Mario Grassi, Michela Abrami, and Gloria Spagnoli. 2021. "Hydrophobically-Modified PEG Hydrogels with Controllable Hydrophilic/Hydrophobic Balance" Polymers 13, no. 9: 1489. https://doi.org/10.3390/polym13091489