Interactions between an Associative Amphiphilic Block Polyelectrolyte and Surfactants in Water: Effect of Charge Type on Solution Properties and Aggregation
Abstract
:1. Introduction
1.1. Models of PE in Solution
1.2. Studies on PESCs
1.3. Aim of the Work
2. Materials and Methods
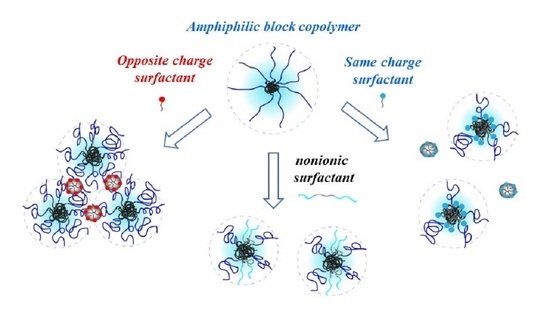
3. Results
3.1. Studied Systems
3.1.1. Same Charge
3.1.2. Opposite Charge
3.1.3. Nonionic Surfactants
3.2. Solution Rheology
3.3. Proposed Model
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, N.; Brettmann, B. Intermolecular Interactions in Polyelectrolyte and Surfactant Complexes in Solution. Polymers 2018, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Gradzielski, M.; Hoffmann, I. Polyelectrolyte-surfactant complexes (PESCs) composed of oppositely charged components. Curr. Opin. Colloid Interface Sci. 2018, 35, 124–141. [Google Scholar] [CrossRef]
- Langevin, D. Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. A review. Adv. Colloid Interface Sci. 2009, 147-148, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Penfold, J.; Thomas, R.; Taylor, D. Polyelectrolyte/surfactant mixtures at the air–solution interface. Curr. Opin. Colloid Interface Sci. 2006, 11, 337–344. [Google Scholar] [CrossRef]
- Berret, J.-F. Controlling electrostatic co-assembly using ion-containing copolymers: From surfactants to nanoparticles. Adv. Colloid Interface Sci. 2011, 167, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kötz, J.; Kosmella, S.; Beitz, T. Self-assembled polyelectrolyte systems. Prog. Polym. Sci. 2001, 26, 1199–1232. [Google Scholar] [CrossRef]
- Iliopoulos, I. Association between hydrophobic polyelectrolytes and surfactants. Curr. Opin. Colloid Interface Sci. 1998, 3, 493–498. [Google Scholar] [CrossRef]
- Langevin, D. Polyelectrolyte and surfactant mixed solutions. Behavior at surfaces and in thin films. Adv. Colloid Interface Sci. 2001, 89–90, 467–484. [Google Scholar] [CrossRef]
- Muthukumar, M. 50th Anniversary Perspective: A Perspective on Polyelectrolyte Solutions. Macromolecules 2017, 50, 9528–9560. [Google Scholar] [CrossRef] [Green Version]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Holm, C.; Rehahn, M.; Oppermann, W.; Ballauff, M. Stiff-Chain Polyelectrolytes. Adv. Polym. Sci. 2004, 166, 1–27. [Google Scholar] [CrossRef]
- Netz, R.R.; Andelman, D. Polyelectrolytes in Solution and at Surfaces. In Encyclopedia of Electrochemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 15 December 2007. [Google Scholar]
- Dobrynin, A.V. Electrostatic Persistence Length of Semiflexible and Flexible Polyelectrolytes. Macromolecules 2005, 38, 9304–9314. [Google Scholar] [CrossRef]
- De Gennes, P.G.; Witten, T.A. Scaling Concepts in Polymer Physics. Phys. Today 1980, 33, 51. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Hydrophobic Polyelectrolytes. Macromolecules 1999, 32, 915–922. [Google Scholar] [CrossRef]
- Essafi, W.; Spiteri, M.-N.; Williams, C.; Boue, F. Hydrophobic Polyelectrolytes in Better Polar Solvent. Structure and Chain Conformation As Seen by SAXS and SANS. Macromolecules 2009, 42, 9568–9580. [Google Scholar] [CrossRef] [Green Version]
- Afolabi, R.O.; Oluyemi, G.F.; Officer, S.; Ugwu, J.O. Hydrophobically associating polymers for enhanced oil recovery – Part A: A review on the effects of some key reservoir conditions. J. Pet. Sci. Eng. 2019, 180, 681–698. [Google Scholar] [CrossRef]
- Wever, D.; Picchioni, F.; Broekhuis, A. Polymers for enhanced oil recovery: A paradigm for structure–property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Migliore, N.; Picchioni, F.; Raffa, P. The effect of macromolecular structure on the rheology and surface properties of amphiphilic random polystyrene-r-poly(meth)acrylate copolymers prepared by RDRP. Soft Matter 2020, 16, 2836–2846. [Google Scholar] [CrossRef] [Green Version]
- Neal, T.J.; Beattie, D.L.; Byard, S.J.; Smith, G.N.; Murray, M.W.; Williams, N.S.J.; Emmett, S.N.; Armes, S.P.; Spain, S.G.; Mykhaylyk, O.O. Self-Assembly of Amphiphilic Statistical Copolymers and Their Aqueous Rheological Properties. Macromolecules 2018, 51, 1474–1487. [Google Scholar] [CrossRef] [Green Version]
- Lauber, L.; Chassenieux, C.; Nicolai, T.; Colombani, O. Highlighting the Role of the Random Associating Block in the Self-Assembly of Amphiphilic Block–Random Copolymers. Macromolecules 2015, 48, 7613–7619. [Google Scholar] [CrossRef]
- Zhang, J.; Farias-Mancilla, B.; Kulai, I.; Hoeppener, S.; Lonetti, B.; Prévost, S.; Ulbrich, J.; Destarac, M.; Colombani, O.; Schubert, U.S.; et al. Effect of Hydrophilic Monomer Distribution on Self-Assembly of a pH-Responsive Copolymer: Spheres, Worms and Vesicles from a Single Copolymer Composition. Angew. Chem. Int. Ed. 2021, 60, 4925–4930. [Google Scholar] [CrossRef]
- Raffa, P.; Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymeric Surfactants: Synthesis, Properties, and Links to Applications. Chem. Rev. 2015, 115, 8504–8563. [Google Scholar] [CrossRef]
- Kimerling, A.S.; Rochefort, W.E.; Bhatia, S.R. Rheology of Block Polyelectrolyte Solutions and Gels: A Review. Ind. Eng. Chem. Res. 2006, 45, 6885–6889. [Google Scholar] [CrossRef]
- Förster, S.; Abetz, V.; Müller, A.H.E. Polyelectrolyte Block Copolymer Micelles. Adv. Polym. Sci. 2012, 166, 173–210. [Google Scholar] [CrossRef]
- Chassenieux, C.; Nicolai, T.; Benyahia, L. Rheology of associative polymer solutions. Curr. Opin. Colloid Interface Sci. 2011, 16, 18–26. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Self-Assembly of Amphiphilic Block Copolymers in Selective Solvents; Springer: Cham, Switzerland, 2016; pp. 27–63. [Google Scholar]
- Raffa, P.; Picchioni, F. Preliminary evaluation of amphiphilic block polyelectrolytes as potential flooding agents for low salinity chemical enhanced oil recovery. J. Pet. Sci. Eng. 2021, 198, 108181. [Google Scholar] [CrossRef]
- Borisov, O.V.; Zhulina, E.B. Effect of Salt on Self-Assembly in Charged Block Copolymer Micelles. Macromolecules 2002, 35, 4472–4480. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V. Theory of Block Polymer Micelles: Recent Advances and Current Challenges. Macromolecules 2012, 45, 4429–4440. [Google Scholar] [CrossRef]
- Chen, W.-L.; Cordero, R.; Tran, H.; Ober, C.K. 50th Anniversary Perspective: Polymer Brushes: Novel Surfaces for Future Materials. Macromolecules 2017, 50, 4089–4113. [Google Scholar] [CrossRef]
- Raffa, P.; Stuart, M.C.; Broekhuis, A.A.; Picchioni, F. The effect of hydrophilic and hydrophobic block length on the rheology of amphiphilic diblock Polystyrene-b-Poly(sodium methacrylate) copolymers prepared by ATRP. J. Colloid Interface Sci. 2014, 428, 152–161. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Sachar, S. Surfactant polymer interactions between strongly interacting cationic surfactants and anionic polyelectrolytes from conductivity and turbidity measurements. Colloid Polym. Sci. 2003, 282, 993–999. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, P.-Y.; Zhao, F.-Y.; An, Q.-F.; Gao, C.-J. Preparation and pervaporation characteristics of novel ethanol permselective polyelectrolyte–surfactant complex membranes. RSC Adv. 2015, 5, 63545–63552. [Google Scholar] [CrossRef]
- Schulze-Zachau, F.; Braunschweig, B. Structure of Polystyrenesulfonate/Surfactant Mixtures at Air–Water Interfaces and Their Role as Building Blocks for Macroscopic Foam. Langmuir 2017, 33, 3499–3508. [Google Scholar] [CrossRef] [PubMed]
- Ritacco, H.; Kurlat, D.; Langevin, D. Properties of Aqueous Solutions of Polyelectrolytes and Surfactants of Opposite Charge: Surface Tension, Surface Rheology, and Electrical Birefringence Studies. J. Phys. Chem. B 2003, 107, 9146–9158. [Google Scholar] [CrossRef]
- Llamas, S.; Fernández-Peña, L.; Akanno, A.; Guzmán, E.; Ortega, V.; Ortega, F.; Csaky, A.G.; Campbell, R.A.; Rubio, R.G. Towards understanding the behavior of polyelectrolyte–surfactant mixtures at the water/vapor interface closer to technologically-relevant conditions. Phys. Chem. Chem. Phys. 2017, 20, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Trabelsi, S.; Guillot, S.; McLoughlin, D.; Langevin, D.; Letellier, A.P.; Turmine, M. Critical Aggregation Concentration in Mixed Solutions of Anionic Polyelectrolytes and Cationic Surfactants. Langmuir 2004, 20, 8496–8503. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Claesson, P.; Bergström, M.; Dėdinaitė, A. Trapped non-equilibrium states in aqueous solutions of oppositely charged polyelectrolytes and surfactants: Effects of mixing protocol and salt concentration. Colloids Surf. A Physicochem. Eng. Asp. 2005, 253, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, S.; Gustavsson, C.; Gudmundsson, C.; Linse, P.; Piculell, L. When Do Water-Insoluble Polyion−Surfactant Ion Complex Salts “Redissolve” by Added Excess Surfactant? Langmuir 2011, 27, 592–603. [Google Scholar] [CrossRef]
- Delisavva, F.; Uchman, M.; Štěpánek, M.; Kereïche, S.; Hordyjewicz-Baran, Z.; Appavou, M.-S.; Procházka, K. Coassembly of Gemini Surfactants with Double Hydrophilic Block Polyelectrolytes Leading to Complex Nanoassemblies. Macromolecules 2017, 50, 8745–8754. [Google Scholar] [CrossRef]
- Bali, K.; Varga, Z.J.; Kardos, A.; Varga, I.; Gilányi, T.; Domján, A.; Wacha, A.; Bóta, A.; Mihály, J.; Mészáros, R. Effect of Dilution on the Nonequilibrium Polyelectrolyte/Surfactant Association. Langmuir 2018, 34, 14652–14660. [Google Scholar] [CrossRef]
- Goswami, M.; Borreguero, J.M.; Pincus, P.A.; Sumpter, B.G. Surfactant-Mediated Polyelectrolyte Self-Assembly in a Polyelectrolyte–Surfactant Complex. Macromolecules 2015, 48, 9050–9059. [Google Scholar] [CrossRef]
- Varga, I.; Campbell, R.A. General Physical Description of the Behavior of Oppositely Charged Polyelectrolyte/Surfactant Mixtures at the Air/Water Interface. Langmuir 2017, 33, 5915–5924. [Google Scholar] [CrossRef]
- Bai, Y.; Shang, X.; Wang, Z.; Zhao, X. Experimental Study on Hydrophobically Associating Hydroxyethyl Cellulose Flooding System for Enhanced Oil Recovery. Energy Fuels 2018, 32, 6713–6725. [Google Scholar] [CrossRef]
- Hansson, P. Self-Assembly of Ionic Surfactants in Polyelectrolyte Solutions: A Model for Mixtures of Opposite Charge. Langmuir 2001, 17, 4167–4180. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, C.; Jiang, H.; Dawadi, M.B.; Vogt, B.D.; Modarelli, D.A.; Zacharia, N.S. Polyelectrolyte-micelle coacervates: Intrapolymer-dominant vs. interpolymer-dominant association, solute uptake and rheological properties. Soft Matter 2019, 15, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, L.; Abelenda-Nuñez, I.; Hernández-Rivas, M.; Ortega, F.; Rubio, R.G.; Guzmán, E. Impact of the bulk aggregation on the adsorption of oppositely charged polyelectrolyte-surfactant mixtures onto solid surfaces. Adv. Colloid Interface Sci. 2020, 282, 102203. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, L.; Temchenko, M.; Colby, R.H. Interactions among Hydrophobically Modified Polyelectrolytes and Surfactants of the Same Charge. Langmuir 2000, 16, 2609–2614. [Google Scholar] [CrossRef]
- Deo, P.; Jockusch, S.; Ottaviani, M.F.; Moscatelli, A.; Turro, N.J.; Somasundaran, P. Interactions of Hydrophobically Modified Polyelectrolytes with Surfactants of the Same Charge. Langmuir 2003, 19, 10747–10752. [Google Scholar] [CrossRef]
- Mafi, A.; Hu, D.; Chou, K.C. Complex Formations between Surfactants and Polyelectrolytes of the Same Charge on a Water Surface. Langmuir 2017, 33, 7940–7946. [Google Scholar] [CrossRef]
- Bassalah, M.E.; Cerdà, J.J.; Sintes, T.; Aschi, A.; Othman, T. Complex between cationic like-charged polyelectrolytes/surfactants systems. Eur. Polym. J. 2017, 96, 55–68. [Google Scholar] [CrossRef]
- Noda, T.; Hashidzume, A.; Morishima, Y. Solution Properties of Micelle Networks Formed by Nonionic Surfactant Moieties Covalently Bound to a Polyelectrolyte: Salt Effects on Rheological Behavior. Langmuir 2000, 16, 5324–5332. [Google Scholar] [CrossRef]
- Galindo-Alvarez, J.; Le, K.-A.; Sadtler, V.; Marchal, P.; Perrin, P.; Tribet, C.; Marie, E.; Durand, A. Enhanced stability of nanoemulsions using mixtures of non-ionic surfactant and amphiphilic polyelectrolyte. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 389, 237–245. [Google Scholar] [CrossRef]
- Fegyver, E.; Mészáros, R. The impact of nonionic surfactant additives on the nonequilibrium association between oppositely charged polyelectrolytes and ionic surfactants. Soft Matter 2014, 10, 1953. [Google Scholar] [CrossRef]
- Chiappisi, L.; Leach, S.D.; Gradzielski, M. Precipitating polyelectrolyte–surfactant systems by admixing a nonionic surfactant–a case of cononsurfactancy. Soft Matter 2017, 13, 4988–4996. [Google Scholar] [CrossRef]
- Lu, Y.; Meng, Z.; Gao, K.; Hou, J.; Wu, H.; Kang, W. Interaction of Amphiphilic Polymers with Medium-Chain Fatty Alcohols to Enhance Rheological Performance and Mobility Control Ability. Energy Fuels 2019, 33, 6273–6282. [Google Scholar] [CrossRef]
- Koutalas, G.; Pispas, S.; Hadjichristidis, N. Micelles of poly(isoprene-b-2-vinylpyridine-b-ethylene oxide) terpolymers in aqueous media and their interaction with surfactants. Eur. Phys. J. E 2004, 15, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Wesley, R.D.; Dreiss, C.A.; Cosgrove, T.; Armes, S.P.; Thompson, L.; Baines, F.L.; Billingham, N.C. Structure of a Hydrophilic−Hydrophobic Block Copolymer and Its Interactions with Salt and an Anionic Surfactant. Langmuir 2005, 21, 4856–4861. [Google Scholar] [CrossRef]
- Jacquin, M.; Muller, P.; Cottet, H.; Crooks, R.; Théodoly, O. Controlling the Melting of Kinetically Frozen Poly(butyl acrylate-b-acrylic acid) Micelles via Addition of Surfactant. Langmuir 2007, 23, 9939–9948. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, W.; Zhao, S.; Shang, Y.; Peng, C.; Wang, H.; Liu, H. Effects of the hydrophilicity or hydrophobicity of the neutral block on the structural formation of a block polyelectrolyte/surfactant complex: A molecular dynamics simulation study. Comput. Condens. Matter 2015, 2, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, D.; Diget, J.S.; Nyström, B.; Gyulai, G.; Mészáros, R.; Gilányi, T.; Varga, I. Response of block copolyelectrolyte complexes to addition of ionic surfactants. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 532, 290–296. [Google Scholar] [CrossRef]
- Borreguero, J.M.; Pincus, P.A.; Sumpter, B.G.; Goswami, M. Unraveling the Agglomeration Mechanism in Charged Block Copolymer and Surfactant Complexes. Macromolecules 2017, 50, 1193–1205. [Google Scholar] [CrossRef]
- Uchman, M.; Hajduová, J.; Vlassi, E.; Pispas, S.; Appavou, M.-S.; Štěpánek, M. Self- and co-assembly of amphiphilic gradient polyelectrolyte in aqueous solution: Interaction with oppositely charged ionic surfactant. Eur. Polym. J. 2015, 73, 212–221. [Google Scholar] [CrossRef]
- Raffa, P.; Brandenburg, P.; Wever, D.A.Z.; Broekhuis, A.A.; Picchioni, F. Polystyrene–Poly(sodium methacrylate) Amphiphilic Block Copolymers by ATRP: Effect of Structure, pH, and Ionic Strength on Rheology of Aqueous Solutions. Macromolecules 2013, 46, 7106–7111. [Google Scholar] [CrossRef] [Green Version]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.J. A comprehensive review of alkaline-surfactant-polymer (ASP) flooding. Asia-Pacific J. Chem. Eng. 2014, 9, 471–489. [Google Scholar] [CrossRef]
- Meijerink, M.; Van Mastrigt, F.; Franken, L.; Stuart, M.C.A.; Picchioni, F.; Raffa, P. Triblock copolymers of styrene and sodium methacrylate as smart materials: Synthesis and rheological characterization. Pure Appl. Chem. 2017, 89, 1641–1658. [Google Scholar] [CrossRef]
- Druetta, P.; Raffa, P.; Picchioni, F. Chemical enhanced oil recovery and the role of chemical product design. Appl. Energy 2019, 252, 113480. [Google Scholar] [CrossRef]
- Noskov, B.A. Dilational surface rheology of polymer and polymer/surfactant solutions. Curr. Opin. Colloid Interface Sci. 2010, 15, 229–236. [Google Scholar] [CrossRef]





| Surfactant | Structure |
|---|---|
| Enordet J1111 |  |
| CTAB |  |
| PEGMe |  |
| Pluronic P123 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffa, P. Interactions between an Associative Amphiphilic Block Polyelectrolyte and Surfactants in Water: Effect of Charge Type on Solution Properties and Aggregation. Polymers 2021, 13, 1729. https://doi.org/10.3390/polym13111729
Raffa P. Interactions between an Associative Amphiphilic Block Polyelectrolyte and Surfactants in Water: Effect of Charge Type on Solution Properties and Aggregation. Polymers. 2021; 13(11):1729. https://doi.org/10.3390/polym13111729
Chicago/Turabian StyleRaffa, Patrizio. 2021. "Interactions between an Associative Amphiphilic Block Polyelectrolyte and Surfactants in Water: Effect of Charge Type on Solution Properties and Aggregation" Polymers 13, no. 11: 1729. https://doi.org/10.3390/polym13111729