A Review on Current Trends of Polymers in Orthodontics: BPA-Free and Smart Materials
Abstract
:1. Introduction
2. Novel BPA-Free Composite Adhesives
3. Clinical Recommendations to Minimize BPA Release
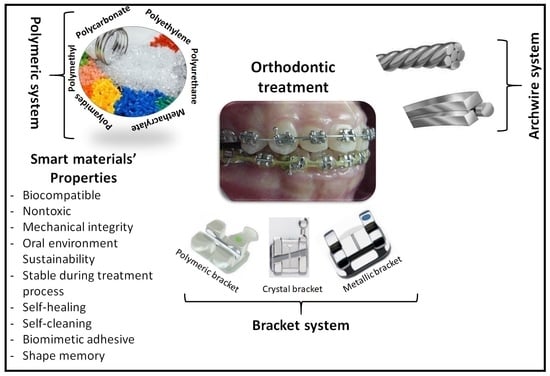
4. Smart Materials in Orthodontics
4.1. Shape-Memory Polymers
4.2. Self-Healing Materials
- (1)
- Dimethacrylate-based polymers to provide a resin network.
- (2)
- Reinforcing filler particles treated with coupling agents to bond the resin to the particles.
- (1)
- Healing powder (HP): strontium fluoroaluminosilicate particles.
- (2)
- Healing liquid (HL): aqueous polyacrylic acid solutions encapsulated in silica microcapsules to prevent premature release during composite preparation.
4.3. Self-Cleaning Materials
4.4. Biomimetic Adhesives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nazir, S.; Khan, M.U.A.; Al-Arjan, W.S.; Abd Razak, S.I.; Javed, A.; Kadir, M.R.A. Nanocomposite Hydrogels for Melanoma Skin Cancer care and Treatment: In-vitro drug delivery, drug release kinetics and anti-Cancer activities. Arab. J. Chem. 2021, 14, 103120. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, A.; Abd Razak, S.I.; Kadir, M.R.A.; Haider, S.; Shah, S.A.; Hasan, A.; Khan, R.; Khan, S.-U.D. Arabinoxylan/graphene-oxide/nHAp-NPs/PVA bio-nano composite scaffolds for fractured bone healing. J. Tissue Eng. Regen. Med. 2021. [Google Scholar] [CrossRef]
- Nienkemper, M.; Willmann, J.H.; Becker, K.; Drescher, D. RFA measurements of survival midpalatal orthodontic mini-implants in comparison to initial healing period. Prog. Orthod. 2020, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Saliba, J.; Modarres, H.P.; Bakhaty, A.; Nasajpour, A.; Mofrad, M.R.; Sanati-Nezhad, A. Micro and nanotechnologies in heart valve tissue engineering. Biomaterials 2016, 103, 278–292. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Haider, A.; Abd Razak, S.I.; Kadir, M.R.A.; Shah, S.A.; Javed, A.; Shakir, I.; Al-Zahrani, A.A. Development of porous, antibacterial and biocompatible GO/n-HAp/bacterial cellulose/β-glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem. 2021, 14, 102924. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Aslam Khan, M.U.; Nazir, S.; Abd Razak, S.I.; Abdul Kadir, M.R. Development of Arabinoxylan-Reinforced Apple Pectin/Graphene Oxide/Nano-Hydroxyapatite Based Nanocomposite Scaffolds with Controlled Release of Drug for Bone Tissue Engineering: In-Vitro Evaluation of Biocompatibility and Cytotoxicity against MC3T3-E1. Coatings 2020, 10, 1120. [Google Scholar] [CrossRef]
- Hasan, A.; Soliman, S.; El Hajj, F.; Tseng, Y.-T.; Yalcin, H.C.; Marei, H.E. Fabrication and in vitro characterization of a tissue engineered PCL-PLLA heart valve. Sci. Rep. 2018, 8, 8187. [Google Scholar] [CrossRef] [PubMed]
- Ariful Islam, M.; Park, T.-E.; Reesor, E.; Cherukula, K.; Hasan, A.; Firdous, J.; Singh, B.; Kang, S.-K.; Choi, Y.-J.; Park, I.-K. Mucoadhesive chitosan derivatives as novel drug carriers. Curr. Pharm. Des. 2015, 21, 4285–4309. [Google Scholar] [CrossRef]
- Kahn, S.; Ehrlich, P.; Feldman, M.; Sapolsky, R.; Wong, S. The jaw epidemic: Recognition, origins, cures, and prevention. BioScience 2020, 70, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Gorrel, C. Veterinary Dentistry for the General Practitioner; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Phulari, B.S. History of Orthodontics; JP Medical Ltd.: London, UK, 2013. [Google Scholar]
- Lyu, X.; Guo, J.; Chen, L.; Gao, Y.; Liu, L.; Pu, L.; Lai, W.; Long, H. Assessment of available sites for palatal orthodontic mini-implants through cone-beam computed tomography. Angle Orthod. 2020, 90, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Ngan, P.; Alkire, R.G.; Fields, H., Jr. Management of space problems in the primary and mixed dentitions. J. Am. Dent. Assoc. 1999, 130, 1330–1339. [Google Scholar] [CrossRef]
- Wexler, A.; Nagappan, A.; Beswerchij, A.; Choi, R. Direct-to-consumer orthodontics: Surveying the user experience. J. Am. Dent. Assoc. 2020, 151, 625–636.e624. [Google Scholar] [CrossRef]
- Kelly, J.E.; Harvey, C.R. An Assessment of the Occlusion of the Teeth of Youths 12–17 Years. Vital Health Stat. 1977, 11, 1–65. [Google Scholar]
- Nessa, K.; Anwar, N.; Ghosh, R.; Mondal, S.; Alam, M.K. Utilization of residual space of deciduous second molar to align teeth in a crowded arch. Bangladesh J. Med. Sci. 2020, 19, 763–768. [Google Scholar] [CrossRef]
- Fadeev, R.; Lanina, A.; Li, P.; Chibisova, M.; Shkarin, V.; Prozorova, N. Method for quantitative assessment of dentofacial anomalies in child and adolescent orthodontics. Arch. Euromed. 2020, 10, 76–81. [Google Scholar] [CrossRef]
- Proffit, W.R.; Fields, H.W., Jr.; Sarver, D.M. Contemporary Orthodontics; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Sutherland, K.; Vanderveken, O.M.; Tsuda, H.; Marklund, M.; Gagnadoux, F.; Kushida, C.A.; Cistulli, P.A. Oral appliance treatment for obstructive sleep apnea: An update. J. Clin. Sleep Med. 2014, 10, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Bishara, S.E.; Staley, R.N. Maxillary expansion: Clinical implications. Am. J. Orthod. Dentofac. Orthop. 1987, 91, 3–14. [Google Scholar] [CrossRef]
- Rahiotis, C.; Eliades, T.; Silikas, N.; Eliades, G. Research on Orthodontic polymers. In Research Methods in Orthodontics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 35–60. [Google Scholar]
- Vicente, A.; Rodríguez-Lozano, F.J.; Martínez-Beneyto, Y.; Jaimez, M.; Guerrero-Gironés, J.; Ortiz-Ruiz, A.J. Biophysical and Fluoride Release Properties of a Resin Modified Glass Ionomer Cement Enriched with Bioactive Glasses. Symmetry 2021, 13, 494. [Google Scholar] [CrossRef]
- Domagała, I.; Gil, L.; Firlej, M.; Pieniak, D.; Selech, J.; Romek, D.; Biedziak, B. Statistical Comparison of the Hardness and Scratch-Resistance of the PMMA Polymers Used in Orthodontic Appliances. Adv. Sci. Technol. Res. J. 2020, 14. [Google Scholar] [CrossRef]
- Ren, J.; Du, Z.; Lin, J.; Chen, Z.; Feng, X. Two Mixed-ligand Coordination Polymers: Treatment Activity on Acute Oral Mucositis during Orthodontic Process by Reducing Inflammatory Response. J. Oleo Sci. 2020, 69, 1051–1059. [Google Scholar] [CrossRef]
- Saito, H.; Miyagawa, Y.; Endo, T. Effects of plastic bracket primer on the shear bond strengths of orthodontic brackets. J. Dent. Sci. 2021, 16, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Poonpiriya, T.; Sawaengkit, P.; Churnjitapirom, P.; Thaweboon, S. Water Sorption and Solubility of Vanillin-Incorporated Self-Curing Orthodontic Polymethylmethacrylate Resin. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2021; pp. 187–191. [Google Scholar]
- Al-Jawoosh, S.; Ireland, A.; Su, B. Characterisation of mechanical and surface properties of novel biomimetic interpenetrating alumina-polycarbonate composite materials. Dent. Mater. 2020, 36, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Ryokawa, H.; Miyazaki, Y.; Fujishima, A.; Miyazaki, T.; Maki, K. The mechanical properties of dental thermoplastic materials in a simulated intraoral environment. Orthod. Waves 2006, 65, 64–72. [Google Scholar] [CrossRef]
- Iliadi, A.; Eliades, T.; Silikas, N.; Eliades, G. Development and testing of novel bisphenol A-free adhesives for lingual fixed retainer bonding. Eur. J. Orthod. 2017, 39, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halimi, A.; Benyahia, H.; Bahije, L.; Adli, H.; Azeroual, M.-F.; Zaoui, F. A systematic study of the release of bisphenol A by orthodontic materials and its biological effects. Int. Orthod. 2016, 14, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Bationo, R.; Jordana, F.; Boileau, M.-J.; Colat-Parros, J. Release of monomers from orthodontic adhesives. Am. J. Orthod. Dentofac. Orthop. 2016, 150, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M. Degradation and formation of bisphenol A in polycarbonate used in dentistry. J. Med. Dent. Sci. 2004, 51, 1–6. [Google Scholar]
- Roongrujimek, P.; Anuwongnukroh, N.; Dechkunakorn, S. Elution Study of Three Light-Cured Orthodontic Adhesives. In Solid State Phenomena; Trans Tech Publications Ltd.: Bäch, Switzerland, 2019; pp. 59–64. [Google Scholar]
- Eliades, T. Orthodontic material applications over the past century: Evolution of research methods to address clinical queries. Am. J. Orthod. Dentofac. Orthop. 2015, 147, S224–S231. [Google Scholar] [CrossRef] [Green Version]
- Bationo, R.; Rouamba, A.; Diarra, A.; Beugré-Kouassi, M.L.A.; Beugré, J.B.; Jordana, F. Cytotoxicity evaluation of dental and orthodontic light-cured composite resins. Clin. Exp. Dent. Res. 2021, 7, 40–48. [Google Scholar] [CrossRef]
- Bhuvaneshwari, V. A Comparative Evaluation of BPA Released from Different Orthodontic Adhesives: An Invitro Study Using Gas Chromatography Coupled with Mass Spectrometry. Ph.D. Thesis, Rajas Dental College and Hospital, Tirunelveli, India, 2020. [Google Scholar]
- Kloukos, D.; Sifakakis, I.; Voutsa, D.; Doulis, I.; Eliades, G.; Katsaros, C.; Eliades, T. BPA qualtitative and quantitative assessment associated with orthodontic bonding in vivo. Dent. Mater. 2015, 31, 887–894. [Google Scholar] [CrossRef] [Green Version]
- Martim, G.C.; Pfeifer, C.S.; Girotto, E.M. Novel urethane-based polymer for dental applications with decreased monomer leaching. Mater. Sci. Eng. C 2017, 72, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Mutar, M.A.; Ghazi, I.F.; Mahdi, M.S. Preparation and characterization of Novel Bis-GMA Dental Nanocomposite and their application as dental material: Mechanical Properties and Water Sorption/volumetric shrinkage. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 012051. [Google Scholar]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Luo, S.; Liu, F.; Yu, B.; He, J. Preparation of low shrinkage stress Bis-GMA free dental resin composites with a synthesized urethane dimethacrylate monomer. J. Biomater. Sci. Polym. Ed. 2019, 30, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Al-Odayni, A.-B.; Alfotawi, R.; Khan, R.; Saeed, W.S.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Synthesis of chemically modified BisGMA analog with low viscosity and potential physical and biological properties for dental resin composite. Dent. Mater. 2019, 35, 1532–1544. [Google Scholar] [CrossRef]
- Ferracane, J. Elution of leachable components from composites. J. Oral Rehabil. 1994, 21, 441–452. [Google Scholar] [CrossRef]
- Xu, Y.; Xepapadeas, A.B.; Koos, B.; Geis-Gerstorfer, J.; Li, P.; Spintzyk, S. Effect of post-rinsing time on the mechanical strength and cytotoxicity of a 3D printed orthodontic splint material. Dent. Mater. 2021, 37, e314–e327. [Google Scholar] [CrossRef]
- Bagley, B.D.; Smith, J.N.; Teeguarden, J.G. Risk assessment of predicted serum concentrations of bisphenol A in children and adults following treatment with dental composite restoratives, dental sealants, or orthodontic adhesives using physiologically based pharmacokinetic modeling. Regul. Toxicol. Pharmacol. 2021, 120, 104839. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, I.-B. Effects of cuspal compliance and radiant emittance of LED light on the cuspal deflection of replicated tooth cavity. Dent. Mater. J. 2021, 2020–2292. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U. New developments of polymeric dental composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Delgado, A.H.; Young, A.M. Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics. Materials 2021, 14, 760. [Google Scholar] [CrossRef]
- Herrera-González, A.M.; González-López, J.A.; Cuevas-Suárez, C.E.; García-Castro, M.A.; Vargas-Ramírez, M. Formulation and evaluation of dental composite resins with allylcarbonate monomer as eluent for Bis-GMA. Polym. Compos. 2018, 39, E342–E350. [Google Scholar]
- Cara, R.; Nicola, C.; Prejmerean, C.; Sava, S.; Băciut, G.; Băciut, M.; Bran, S.; Bondor, C.; Prodan, D.; Moldovan, M. Influence of Bis-GMA Derivative Monomer-Based Particulate Composite Resins on the Cuspal Deformation and Microleakage of Restored Teeth. Part. Sci. Technol. 2010, 28, 191–206. [Google Scholar] [CrossRef]
- Santos, B.M.; Pithon, M.M.; Ruellas, A.C.; Sant’Anna, E.F. Shear bond strength of brackets bonded with hydrophilic and hydrophobic bond systems under contamination. Angle Orthod. 2010, 80, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O. Biocompatibility of Ti surfaces with different coatings designed for orthodontic treatment. Ph.D. Thesis, Medical University of Vienna, Vienna, Austria, March 2020. [Google Scholar]
- Quassem, M.A.; Abdelrahman, M.A.S. Effect of Silanized and Non-silanized Glass Fiber on Water Sorption, Solubility and Roughness of Heat Cured Acrylic Prosthodontic and Orthodontic Appliances. Egypt. Dent. J. 2020, 66, 1133–1141. [Google Scholar] [CrossRef]
- Merani, V.; Pulgaonkar, R.; Jethe, S.; Rahalkar, J.S.; Deshmukh, S.; Dongre, S. Evaluation of orthodontic shear peel-band strength, adhesive remnants, site of bond failure and solubility of three commonly used cements when applied to pre-fabricated and customized bands: An in-vitro study. J. Adv. Med. Dent. Sci. Res. 2021, 9, 5–10. [Google Scholar]
- Hassan, A.A.; Jameel, A.; Nahidh, M.; Hamid, D. Evaluation ofásome mechanical and physical properties ofádifferent types ofáinjectable polymer materials used as aábase for removable orthodontic appliances. J. Stomatol. 2019, 72, 215–221. [Google Scholar]
- Cebe, M.A.; Cebe, F.; Cengiz, M.F.; Cetin, A.R.; Arpag, O.F.; Ozturk, B. Elution of monomer from different bulk fill dental composite resins. Dent. Mater. 2015, 31, e141–e149. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Wojnowska-Baryła, I.; Cydzik-Kwiatkowska, A. Bisphenol A Removal from Water and Wastewater; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Schnettler, R.; Barbeck, M. Additive manufacturing for guided bone regeneration: A perspective for alveolar ridge augmentation. Int. J. Mol. Sci. 2018, 19, 3308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, L.; Wang, Y.; Wang, L.; Zhang, H.; Na, H.; Zhang, Z. Synthesis and characterization of a novel resin monomer with low viscosity. J. Dent. 2017, 59, 11–17. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, G.; Sun, H.; Jiang, H.; Zhao, C.; Xu, D.; Li, H.; Sun, X.; Na, H. Preparation, characterization and thermal properties of tetramethylbisphenol F epoxy resin and mixed systems. Polym. Int. 2012, 61, 565–570. [Google Scholar] [CrossRef]
- Zhao, B.; Van Der Mei, H.C.; Subbiahdoss, G.; de Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, X.; Zhang, Q.; Zhu, M. Strong and bioactive dental resin composite containing poly (Bis-GMA) grafted hydroxyapatite whiskers and silica nanoparticles. Compos. Sci. Technol. 2014, 101, 86–93. [Google Scholar] [CrossRef]
- Stein, P.S.; Sullivan, J.; Haubenreich, J.E.; Osborne, P.B. Composite resin in medicine and dentistry. J. Long-Term Eff. Med. Implant. 2005, 15, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Wille, S.; Hölken, I.; Haidarschin, G.; Adelung, R.; Kern, M. Biaxial flexural strength of new Bis-GMA/TEGDMA based composites with different fillers for dental applications. Dent. Mater. 2016, 32, 1073–1078. [Google Scholar] [CrossRef]
- Ruyter, I.E. Methacrylate-based polymeric dental materials: Conversion and related properties: Summary and review. Acta Odontol. Scand. 1982, 40, 359–376. [Google Scholar] [CrossRef]
- Anseth, K.; Newman, S.; Bowman, C. Polymeric dental composites: Properties and reaction behavior of multimethacrylate dental restorations. Biopolymers II 1995, 122, 177–217. [Google Scholar]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Sunarso; Tsuchiya, A.; Fukuda, N.; Toita, R.; Tsuru, K.; Ishikawa, K. Effect of micro-roughening of poly (ether ether ketone) on bone marrow derived stem cell and macrophage responses, and osseointegration. J. Biomater. Sci. Polym. Ed. 2018, 29, 1375–1388. [Google Scholar] [CrossRef]
- Rodrıguez, J.; Martın, A.; Fernández, R.; Fernández, J. An experimental study of the wear performance of NiCrBSi thermal spray coatings. Wear 2003, 255, 950–955. [Google Scholar] [CrossRef]
- Heimer, S.; Schmidlin, P.R.; Roos, M.; Stawarczyk, B. Surface properties of polyetheretherketone after different laboratory and chairside polishing protocols. J. Prosthet. Dent. 2017, 117, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Wischke, C.; Neffe, A.T.; Steuer, S.; Lendlein, A. Evaluation of a degradable shape-memory polymer network as matrix for controlled drug release. J. Control. Release 2009, 138, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Cho, J.W. Application of shape memory polyurethane in orthodontic. J. Mater. Sci. Mater. Med. 2010, 21, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Larock, R.C. New soybean oil–styrene–divinylbenzene thermosetting copolymers. v. shape memory effect. J. Appl. Polym. Sci. 2002, 84, 1533–1543. [Google Scholar] [CrossRef]
- Hernández, A.R.; Contreras, O.C.; Acevedo, J.C.; Moreno, L.G.N. Poly(ε-caprolactone) degradation under acidic and alkaline conditions. Am. J. Polym. Sci. 2013, 3, 70. [Google Scholar]
- Jones, A.; Rule, J.; Moore, J.; Sottos, N.; White, S. Life extension of self-healing polymers with rapidly growing fatigue cracks. J. R. Soc. Interface 2007, 4, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Huyang, G.; Debertin, A.E.; Sun, J. Design and development of self-healing dental composites. Mater. Des. 2016, 94, 295–302. [Google Scholar] [CrossRef] [Green Version]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Wu, J.; Weir, M.D.; Melo, M.A.S.; Strassler, H.E.; Xu, H.H. Effects of water-aging on self-healing dental composite containing microcapsules. J. Dent. 2016, 47, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Yahyazadehfar, M.; Huyang, G.; Wang, X.; Fan, Y.; Arola, D.; Sun, J. Durability of self-healing dental composites: A comparison of performance under monotonic and cyclic loading. Mater. Sci. Eng. C 2018, 93, 1020–1026. [Google Scholar] [CrossRef]
- Mitwalli, H.; Alsahafi, R.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.; Melo, M.A.S. Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials. Bioengineering 2020, 7, 83. [Google Scholar] [CrossRef]
- Taylor, R.; Maryan, C.; Verran, J. Retention of oral microorganisms on cobalt-chromium alloy and dental acrylic resin with different surface finishes. J. Prosthet. Dent. 1998, 80, 592–597. [Google Scholar] [CrossRef]
- Nishimoto, S.; Bhushan, B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 2013, 3, 671–690. [Google Scholar] [CrossRef]
- Okada, A.; Nikaido, T.; Ikeda, M.; Okada, K.; Yamauchi, J.; Foxton, R.M.; Sawada, H.; Tagami, J.; Matin, K. Inhibition of biofilm formation using newly developed coating materials with self-cleaning properties. Dent. Mater. J. 2008, 27, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, D.; van den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A Recovery Utilizat. Environ. Eff. 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Berengueres, J.; Saito, S.; Tadakuma, K. Structural properties of a scaled gecko foot-hair. Bioinspir. Biomim. 2007, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Eliades, T. Future of bonding. In Orthodontic Applications of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 267–271. [Google Scholar]
- Purushothaman, B.; Jayachandran, A.; Krishnan, S.; Abraham, N. Bond with the best. Dent. Bites 2018, 5, 32–40. [Google Scholar]
- Zhao, P.; Wei, K.; Feng, Q.; Chen, H.; Wong, D.S.H.; Chen, X.; Wu, C.-C.; Bian, L. Mussel-mimetic hydrogels with defined cross-linkers achieved via controlled catechol dimerization exhibiting tough adhesion for wet biological tissues. Chem. Commun. 2017, 53, 12000–12003. [Google Scholar] [CrossRef] [PubMed]
- James, V.; Madhubala, M.M.; Devarajan, S.S.; Mahalaxmi, S.; Sathyakumar, S. Evaluation of Degree of Conversion, Resin-Dentin Bond Strength, and Durability of Polydopamine Incorporated Total Etch Adhesive System. Front. Dent. 2020. [Google Scholar] [CrossRef]
- Saxer, S.; Portmann, C.; Tosatti, S.; Gademann, K.; Zurcher, S.; Textor, M. Surface assembly of catechol-functionalized poly(l-lysine)-graft-poly(ethylene glycol) copolymer on titanium exploiting combined electrostatically driven self-organization and biomimetic strong adhesion. Macromolecules 2010, 43, 1050–1060. [Google Scholar] [CrossRef]
- Hui, C.-Y.; Glassmaker, N.; Tang, T.; Jagota, A. Design of biomimetic fibrillar interfaces: 2. Mechanics of enhanced adhesion. J. R. Soc. Interface 2004, 1, 35–48. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, R.; Aslam Khan, M.U.; Abdullah, A.M.; Abd Razak, S.I. A Review on Current Trends of Polymers in Orthodontics: BPA-Free and Smart Materials. Polymers 2021, 13, 1409. https://doi.org/10.3390/polym13091409
Hassan R, Aslam Khan MU, Abdullah AM, Abd Razak SI. A Review on Current Trends of Polymers in Orthodontics: BPA-Free and Smart Materials. Polymers. 2021; 13(9):1409. https://doi.org/10.3390/polym13091409
Chicago/Turabian StyleHassan, Rozita, Muhammad Umar Aslam Khan, Abdul Manaf Abdullah, and Saiful Izwan Abd Razak. 2021. "A Review on Current Trends of Polymers in Orthodontics: BPA-Free and Smart Materials" Polymers 13, no. 9: 1409. https://doi.org/10.3390/polym13091409