Dual Plasticizer/Thermal Stabilizer Effect of Epoxidized Chia Seed Oil (Salvia hispanica L.) to Improve Ductility and Thermal Properties of Poly(Lactic Acid)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Mechanical and Thermal Characterization
2.3. Morphology Characterization
2.4. Disintegration under Composting Conditions
2.5. Migration of Plasticizer by Solvent Extraction Test
3. Results and Discussion
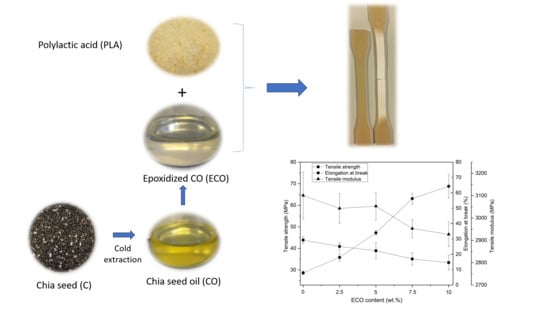
3.1. Effect of ECO in PLA on the Mechanical Properties
3.2. Effect of ECO in PLA on the Thermomechanical Properties of PLA
3.3. Effect of ECO in PLA on the Thermal Properties of PLA
3.4. Disintegration under Composting Conditions
3.5. Migration of ECO by Solvent Extraction Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Europe, P. Plastics—The Facts An analysis of European plastics production, demand and waste. Plastics Europe, Brussels. Available online: https://www.plasticseurope.org/download_file/force/4261/181 (accessed on 15 March 2021).
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef] [Green Version]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in Four Estuarine Rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Rani, M.; Lee, J.; Shim, W.J. A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples. Mar. Pollut. Bull. 2015, 93, 202–209. [Google Scholar] [CrossRef]
- Râpă, M.; Miteluţ, A.C.; Tănase, E.E.; Grosu, E.; Popescu, P.; Popa, M.E.; Rosnes, J.T.; Sivertsvik, M.; Darie-Niţă, R.N.; Vasile, C. Influence of chitosan on mechanical, thermal, barrier and antimicrobial properties of PLA-biocomposites for food packaging. Compos. Part. B Eng. 2016, 102, 112–121. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of poly (lactic) acid and starch for biodegradable food packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Ivonkovic, A.; Zeljko, K.; Talic, S.; Lasic, M. Biodegradable packaging in the food industry. J. Food Saf. Food Qual. 2017, 68, 26–38. [Google Scholar]
- Musioł, M.; Sikorska, W.; Adamus, G.; Janeczek, H.; Kowalczuk, M.; Rydz, J. (Bio)degradable polymers as a potential material for food packaging: Studies on the (bio)degradation process of PLA/(R,S)-PHB rigid foils under industrial composting conditions. Eur. Food Res. Technol. 2016, 242, 815–823. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Vink, E.T.; Rábago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [Green Version]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers-A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Malathi, A.; Santhosh, K.; Nidoni, U. Recent trends of biodegradable polymer: Biodegradable films for food packaging and application of nanotechnology in biodegradable food packaging. Curr. Trends Technol. Sci. 2014, 3, 73–79. [Google Scholar]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly (lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayan, M.; Azizi, H.; Ghasemi, I.; Karrabi, M. Influence of modified starch and nanoclay particles on crystallization and thermal degradation prop-erties of cross-linked poly (lactic acid). J. Polym. Res. 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Lemmouchi, Y.; Murariu, M.; Dos Santos, A.M.; Amass, A.J.; Schacht, E.; Dubois, P. Plasticization of poly(lactide) with blends of tributyl citrate and low molecular weight poly(d,l-lactide)-b-poly(ethylene glycol) copolymers. Eur. Polym. J. 2009, 45, 2839–2848. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Pluta, M.; Piorkowska, E.; Krasnikova, N. Plasticization of polylactide with block copolymers of ethylene glycol and propylene glycol. J. Appl. Polym. Sci. 2012, 125, 4292–4301. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Fenollar, O.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of Mechanical Ductile Properties of Poly(3-hydroxybutyrate) by Using Vegetable Oil Derivatives. Macromol. Mater. Eng. 2017, 302, 1600330. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wang, Y.; Ruan, J.; Zhang, J.; Sun, C. Recent advances in analysis of phthalate esters in foods. TRAC Trends Anal. Chem. 2015, 72, 10–26. [Google Scholar] [CrossRef]
- Khosravi, K.; Price, G.W. Determination of phthalates in soils and biosolids using accelerated solvent extraction coupled with SPE cleanup and GC–MS quantification. Microchem. J. 2015, 121, 205–212. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Z.; Chen, G.; Zhang, W.; Cai, Y.; Kong, R.; Wang, X.; Suo, Y.; You, J. Determination of phthalate esters in environmental water by magnetic Zeolitic Imidazolate Framework-8 solid-phase extraction coupled with high-performance liquid chromatography. J. Chromatogr. A 2015, 1409, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M. Polymer Chemistry: An Introduction; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Plasticized poly(lactic acid) with low molecular weight poly(ethylene glycol): Mechanical, thermal, and morphology properties. J. Appl. Polym. Sci. 2013, 130, 4576–4580. [Google Scholar] [CrossRef]
- Tsou, C.-H.; Gao, C.; De Guzman, M.; Wu, D.-Y.; Hung, W.-S.; Yuan, L.; Suen, M.-C.; Yeh, J.-T. Preparation and characterization of poly(lactic acid) with adipate ester added as a plasticizer. Polym. Polym. Compos. 2018, 26, 446–453. [Google Scholar] [CrossRef]
- Shirai, M.; Grossmann, M.; Mali, S.; Yamashita, F.; Garcia, P.; Müller, C. Development of biodegradable flexible films of starch and poly(lactic acid) plasticized with adipate or citrate esters. Carbohydr. Polym. 2013, 92, 19–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.-J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part. A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-Q.; Qu, J.-P. Mechanical and rheological properties of epoxidized soybean oil plasticized poly(lactic acid). J. Appl. Polym. Sci. 2009, 112, 3185–3191. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Arrieta, M.; López-Martínez, J.; Samper, M. Improvement of PLA film ductility by plasticization with epoxidized karanja oil. Polym. Degrad. Stab. 2020, 179, 109259. [Google Scholar] [CrossRef]
- Balart, J.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sánchez-Nacher, L. Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Compos. Part. B Eng. 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef]
- Al-Mulla, E.A.J.; Yunus, W.M.Z.W.; Ibrahim, N.A.B.; Rahman, M.Z.A. Properties of epoxidized palm oil plasticized polytlactic acid. J. Mater. Sci. 2010, 45, 1942–1946. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized Vegetable Oils Plasticized Poly(lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbonell-Verdu, A.; Samper, M.D.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Plasticization effect of epoxidized cottonseed oil (ECSO) on poly(lactic acid). Ind. Crop. Prod. 2017, 104, 278–286. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Montanes, N.; Lopez-Martinez, J.; Balart, R. Plasticization effects of epoxidized vegetable oils on mechanical properties of poly(3-hydroxybutyrate). Polym. Int. 2016, 65, 1157–1164. [Google Scholar] [CrossRef]
- Fenollar, O.; Sanchez-Nacher, L.; Garcia-Sanoguera, D.; López, J.; Balart, R. The effect of the curing time and temperature on final properties of flexible PVC with an epoxidized fatty acid ester as natural-based plasticizer. J. Mater. Sci. 2009, 44, 3702–3711. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Garcia-Sanoguera, D.; Jordá-Vilaplana, A.; Sanchez-Nacher, L.; Balart, R. A new biobased plasticizer for poly(vinyl chloride) based on epoxidized cottonseed oil. J. Appl. Polym. Sci. 2016, 133, 43642/1–43642/10. [Google Scholar] [CrossRef]
- Thuy, N.T.; Nam, B.X.; Duc, V.M. Study to improve the properties of polylactic acid by epoxidized crude rubber seed oil. Vietnam. J. Chem. 2019, 57, 735–740. [Google Scholar] [CrossRef]
- Thakur, S.; Cisneros-Lopez, E.O.; Pin, J.-M.; Misra, M.; Mohanty, A.K. Green Toughness Modifier from Downstream Corn Oil in Improving Poly(lactic acid) Performance. ACS Appl. Polym. Mater. 2019, 1, 3396–3406. [Google Scholar] [CrossRef]
- Rizzuto, M.; Mugica, A.; Zubitur, M.; Caretti, D.; Müller, A.J. Plasticization and anti-plasticization effects caused by poly(lactide-ran-caprolactone) addition to double crystalline poly(l-lactide)/poly(ε-caprolactone) blends. CrystEngComm 2016, 18, 2014–2023. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of sugar Palm-derived cellulose reinforcement on the mechanical and water barrier properties of sugar palm starch biocomposite films. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Al-Mulla, E.A.J.; Ibrahim, N.A.B.; Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W. Effect of epoxidized palm oil on the mechanical and morphological properties of a PLA–PCL blend. Res. Chem. Intermed. 2014, 40, 689–698. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Boronat, T.; Quiles-Carrillo, L.; Fenollar, O.; Dominici, F.; Torre, L. Valorization of Cotton Industry Byproducts in Green Composites with Polylactide. J. Polym. Environ. 2020, 28, 2039–2053. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Montanes, N.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil as biobased plasticizer in poly(lactic acid)-based formulations. Polym. Int. 2017, 66, 882–891. [Google Scholar] [CrossRef]
- Yong, A.X.; Sims, G.D.; Gnaniah, S.J.; Ogin, S.L.; Smith, P.A. Heating rate effects on thermal analysis measurement of T g in composite materials. Advanced Manufacturing. Polym. Compos. Sci. 2017, 3, 43–51. [Google Scholar]
- Dobircau, L.; Delpouve, N.; Herbinet, R.; Domenek, S.; Le Pluart, L.; Delbreilh, L.; Ducruet, V.; Dargent, E. Molecular mobility and physical ageing of plasticized poly(lactide). Polym. Eng. Sci. 2015, 55, 858–865. [Google Scholar] [CrossRef]
- Silverajah, V.S.G.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Abu Hassan, H.; Woei, C.B. A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend. Int. J. Mol. Sci. 2012, 13, 5878–5898. [Google Scholar] [CrossRef] [Green Version]
- Alam, J.; Alam, M.; Raja, M.; Abduljaleel, Z.; Dass, L.A. MWCNTs-Reinforced Epoxidized Linseed Oil Plasticized Polylactic Acid Nanocomposite and Its Electroactive Shape Memory Behaviour. Int. J. Mol. Sci. 2014, 15, 19924–19937. [Google Scholar] [CrossRef]
- Jebrane, M.; Cai, S.; Panov, D.; Yang, X.; Terziev, N. Synthesis and characterization of new vinyl acetate grafting onto epoxidized linseed oil in aqueous media. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Rao, K.C.; Kumar, D. Preparation and characterization of microcapsules containing linseed oil and its use in self-healing coatings. Prog. Org. Coat. 2008, 63, 72–78. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined Effect of Poly(hydroxybutyrate) and Plasticizers on Polylactic acid Properties for Film Intended for Food Packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Arrieta, M.; de Dicastillo, C.L.; Garrido, L.; Roa, K.; Galotto, M. Electrospun PVA fibers loaded with antioxidant fillers extracted from Durvillaea antarctica algae and their effect on plasticized PLA bionanocomposites. Eur. Polym. J. 2018, 103, 145–157. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Montanes, N.; Fenollar, O.; García-Sanoguera, D.; Balart, R. Development and optimization of renewable vinyl plastisol/wood flour composites exposed to ultraviolet radiation. Mater. Des. 2016, 108, 648–658. [Google Scholar] [CrossRef]
- Balart, J.F.; Montanes, N.; Fombuena, V.; Boronat, T.; Sánchez-Nacher, L. Disintegration in Compost Conditions and Water Uptake of Green Composites from Poly(Lactic Acid) and Hazelnut Shell Flour. J. Polym. Environ. 2017, 26, 701–715. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.; Kittikorn, T.; Strömberg, E.; Martínez-Felipe, A.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Hydrothermal ageing of polylactide/sisal biocomposites. Studies of water absorption behaviour and Physico-Chemical performance. Polym. Degrad. Stab. 2014, 108, 212–222. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Ogami, A.; Okamoto, M.; Ueda, K. New Polylactide/Layered Silicate Nanocomposite: Nanoscale Control Over Multiple Properties. Macromol. Rapid Commun. 2002, 23, 943–947. [Google Scholar] [CrossRef]
- Conn, R.; Kolstad, J.; Borzelleca, J.; Dixler, D.; Filer, L.; Ladu, B.; Pariza, M. Safety assessment of polylactide (PLA) for use as a food-contact polymer. Food Chem. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef]
- Till, D.; Reid, R.; Schwartz, P.; Sidman, K.; Valentine, J.; Whelan, R. Plasticizer migration from polyvinyl chloride film to solvents and foods. Food Chem. Toxicol. 1982, 20, 95–104. [Google Scholar] [CrossRef]
- Wang, X.; Song, M.; Liu, S.; Wu, S.; Thu, A.M. Analysis of phthalate plasticizer migration from PVDC packaging materials to food simulants using molecular dynamics simulations and artificial neural network. Food Chem. 2020, 317, 126465. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, M. Migration of Monomeric and Polymeric PVC Plasticizers. Chromatogr. Sustain. Polym. Mater. 2008, 211, 159–185. [Google Scholar] [CrossRef]










| Reference | Parts by Weight (wt.%) | |
|---|---|---|
| PLA | ECO | |
| PLA | 100 | 0 |
| PLA_2.5%ECO | 97.5 | 2.5 |
| PLA_5%ECO | 95 | 5 |
| PLA_7.5%ECO | 92.5 | 7.5 |
| PLA_10%ECO | 90 | 10 |
| Reference | VST (°C) | HDT (°C) |
|---|---|---|
| PLA | 56.6 ± 1.5 | 52.8 ± 0.5 |
| PLA_2.5%ECO | 55 ± 1.2 | 51.8 ± 0.6 |
| PLA_5%ECO | 53.2 ± 1.3 | 50.4 ± 0.4 |
| PLA_7.5%ECO | 52.2 ± 1.1 | 50.4 ± 0.6 |
| PLA_10%ECO | 52.2 ± 1.4 | 50.2 ± 0.4 |
| Reference | Tg (°C) 1 | Tcc (°C) 2 | Tm (°C) 4 | Hm (J·g−1) 5 | ||
|---|---|---|---|---|---|---|
| PLA | 62.0 | 119.4 | 8.00 | 150.0 | 15.0 | 7.5 |
| PLA_2.5%ECO | 60.0 | 117.3 | 12.5 | 149.7 | 19.6 | 7.9 |
| PLA_5%ECO | 59.2 | 117.7 | 10.9 | 149.0 | 18.4 | 8.5 |
| PLA_7.5%ECO | 56.3 | 118.0 | 11.6 | 148.2 | 20.3 | 10.1 |
| PLA_10%ECO | 56.8 | 118.5 | 8.4 | 148.4 | 18.0 | 11.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez-Candela, I.; Ferri, J.M.; Cardona, S.C.; Lora, J.; Fombuena, V. Dual Plasticizer/Thermal Stabilizer Effect of Epoxidized Chia Seed Oil (Salvia hispanica L.) to Improve Ductility and Thermal Properties of Poly(Lactic Acid). Polymers 2021, 13, 1283. https://doi.org/10.3390/polym13081283
Dominguez-Candela I, Ferri JM, Cardona SC, Lora J, Fombuena V. Dual Plasticizer/Thermal Stabilizer Effect of Epoxidized Chia Seed Oil (Salvia hispanica L.) to Improve Ductility and Thermal Properties of Poly(Lactic Acid). Polymers. 2021; 13(8):1283. https://doi.org/10.3390/polym13081283
Chicago/Turabian StyleDominguez-Candela, Ivan, Jose Miguel Ferri, Salvador Cayetano Cardona, Jaime Lora, and Vicent Fombuena. 2021. "Dual Plasticizer/Thermal Stabilizer Effect of Epoxidized Chia Seed Oil (Salvia hispanica L.) to Improve Ductility and Thermal Properties of Poly(Lactic Acid)" Polymers 13, no. 8: 1283. https://doi.org/10.3390/polym13081283