Synthesis and Thermal Degradation Study of Polyhedral Oligomeric Silsesquioxane (POSS) Modified Phenolic Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
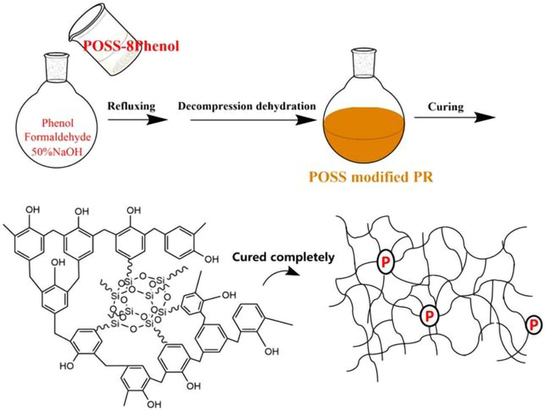
2.2. Sample Preparation
2.3. Sample Characterization
3. Results and Discussion
3.1. Synthesis of Modified Phenolic Resin
3.2. Thermogravimetric Analysis
3.3. Degradation Volatiles’ Analysis
3.4. Degradation Residue Analysis
4. Conclusions
- The introduction of POSS makes phenolic resin show better thermal stability under air. The residual char yield of POSS modified phenolic resin at 800 °C is 14.73% higher than that of phenolic resin. However, under argon atmosphere, the residual char yield of POSS modified phenolic resin at 800 °C is only 6.95% higher than that of phenolic resin.
- Under argon, phenolic resin releases the degradation volatiles at around 580–610 °C, while POSS modified phenolic resin does not reach the maximum speed until 630 °C. The corresponding volatiles evolutions under air are more complex than those under argon. Under air, the volatiles release rate of phenolic resin is the fastest at around 480–520 °C, while volatiles produced by the thermal degradation of POSS modified phenolic resin reach the maximum peaks at 540–610 °C.
- The degradation processes of both phenolic resins are carbonization processes, and the solid phase in the residual products is mainly composed of char. In addition to the char, crystalline SiO2 is detected in the residue of POSS modified phenolic resin after degradation under air. The existence of SiO2 is mainly attributed to the reaction of POSS with oxygen at high temperatures, and the existence of SiO2 can effectively improve the high temperature oxidation resistance of phenolic resin.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.; Chen, W.; Whang, W.; Chen, C. Characteristics of Thermosetting Polymer Nanocomposites: Siloxane-Imide-Containing Benzoxazine with Silsesquioxane Epoxy Resins. Polymers 2020, 12, 2510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, S.; Liu, Y. The effect of titanium incorporation on the thermal stability of phenol-formaldehyde resin and its carbonization microstructure. Polym. Degrad. Stab. 2013, 98, 514–518. [Google Scholar] [CrossRef]
- Abdalla, M.O.; Ludwick, A.; Mitchell, T. Boron-modified phenolic resins for high performance applications. Polymer 2003, 44, 7353–7359. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Z.; Guo, Z. Bionic boron/silicon-modified phenolic resin system with multifunctional groups: Synthesis, thermal properties and ablation mechanism. Biosurf. Biotribol. 2018, 4, 85–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, S.; Yoonessi, M.; Liang, K.; Pittman, C.U. Phenolic resin–trisilanolphenyl polyhedral oligomeric silsesquioxane (POSS) hybrid nanocomposites: Structure and properties. Polymer 2006, 47, 2984–2996. [Google Scholar] [CrossRef]
- Wang, H.; Eberhardt, T.L.; Wang, C.; Gao, S.; Pan, H. Demethylation of Alkali Lignin with Halogen Acids and Its Application to Phenolic Resins. Polymers 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Sun, Y. Strong Effect of Process Parameters on the Properties of Boron-Containing Phenolic Resins with High Char Yield. Appl. Sci. 2020, 10, 1408. [Google Scholar] [CrossRef] [Green Version]
- Acocella, M.R.; Vittore, A.; Maggio, M.; Guerra, G.; Giannini, L.; Tadiello, L. Graphene Oxide and Oxidized Carbon Black as Catalyst for Crosslinking of Phenolic Resins. Polymers 2019, 11, 1330. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Chen, F.; Li, S.; Du, Y.; Luo, Z.; Sun, Y.; Li, H.; Zhao, T. Synthesis of Silicon Hybrid Phenolic Resins with High Si-Content and Nanoscale Phase Separation Structure. Processes 2020, 8, 1129. [Google Scholar] [CrossRef]
- Kaynakli, O. A review of the economical and optimum thermal insulation thickness for building applications. Renew. Sustain. Energy Rev. 2012, 16, 415–425. [Google Scholar] [CrossRef]
- Lin, H.; Wan, X.; Jiang, X.; Wang, Q.; Yin, J. A Nanoimprint Lithography Hybrid Photoresist Based on the Thiol-Ene System. Adv. Funct. Mater. 2011, 21, 2960–2967. [Google Scholar] [CrossRef]
- Rivera Lopez, M.Y.; Lambas, J.M.; Stacey, J.P.; Gamage, S.; Suliga, A.; Viquerat, A.; Scarpa, F.; Hamerton, I. Development of Cycloaliphatic Epoxy-POSS Nanocomposite Matrices with Enhanced Resistance to Atomic Oxygen. Molecules 2020, 25, 1483. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Colonna, S.; Fina, A.; Monticelli, O. Polyhedral Oligomeric Silsesquioxane (POSS) Surface Grafting: A Novel Method to Enhance Polylactide Hydrolysis Resistance. Nanomaterials 2019, 9, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zheng, S.; Nie, K. Morphology and Thermomechanical Properties of Organic−Inorganic Hybrid Composites Involving Epoxy Resin and an Incompletely Condensed Polyhedral Oligomeric Silsesquioxane. Macromolecules 2005, 38, 5088–5097. [Google Scholar] [CrossRef]
- Lungu, A.; Ghitman, J.; Cernencu, A.I.; Serafim, A.; Florea, N.M.; Vasile, E.; Iovu, H. POSS-containing hybrid nanomaterials based on thiol-epoxy click reaction. Polymer 2018, 145, 324–333. [Google Scholar] [CrossRef]
- Lichtenhan, J.; Haddad, T.S.; Schwab, J.; Carr, M.J.; Chaffee, K.P.; Mather, P. The next generation of silicon-based plastics: Polyhedral oligomeric silsesquioxane (POSS) nanocomposites. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 1998, 39, 489–490. [Google Scholar]
- Lee, Y.; Kuo, S.; Su, Y.; Chen, J.; Tu, C.; Chang, F. Syntheses, thermal properties, and phase morphologies of novel benzoxazines functionalized with polyhedral oligomeric silsesquioxane (POSS) nanocomposites. Polymer 2004, 45, 6321–6331. [Google Scholar] [CrossRef]
- Li, S.; Han, Y.; Chen, F.; Luo, Z.; Li, H.; Zhao, T. The effect of structure on thermal stability and anti-oxidation mechanism of silicone modified phenolic resin. Polym. Degrad. Stab. 2016, 124, 68–76. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Kuo, S. Highly Thermal Stable Phenolic Resin Based on Double-Decker-Shaped POSS Nanocomposites for Supercapacitors. Polymers 2020, 12, 2151. [Google Scholar] [CrossRef]
- Dong, Y.; He, J.; Yang, R. Phenolic resin/polyhedral oligomeric silsesquioxane (POSS) composites: Mechanical, ablative, thermal, and flame retardant properties. Polym. Adv. Technol. 2019, 30, 2075–2085. [Google Scholar] [CrossRef]
- Purse, M.; Edmund, G.; Hall, S.; Howlin, B.; Hamerton, I.; Till, S. Reactive Molecular Dynamics Study of the Thermal Decomposition of Phenolic Resins. J. Compos. Sci. 2019, 3, 32. [Google Scholar] [CrossRef] [Green Version]
- Trick, K.A.; Saliba, T.E. Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite. Carbon 1995, 33, 1509–1515. [Google Scholar] [CrossRef]
- Yoshitake, M.; Kamiyama, Y.; Nishi, K.; Yoshimoto, N.; Morita, M.; Sakai, T.; Fujii, K. Defect-free network formation and swelling behavior in ionic liquid-based electrolytes of tetra-arm polymers synthesized using a Michael addition reaction. Phys. Chem. Chem. Phys. 2017, 19, 29984–29990. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater. 2013, 26, 724–744. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Chen, Y.; Zhang, L.; Ou, J.; Shen, Y.; Ye, M. Thiol-radical-mediated polymerization for preparation of POSS-containing polyacrylate monoliths in capillary liquid chromatography. Talanta 2018, 190, 62–69. [Google Scholar] [CrossRef]
- Frayne, S.H.; Murthy, R.R.; Northrop, B.H. Investigation and Demonstration of Catalyst/Initiator-Driven Selectivity in Thiol-Michael Reactions. J. Org. Chem. 2017, 82, 7946–7956. [Google Scholar] [CrossRef] [PubMed]
- Emrah, A. Allylamino diphenylphosphine oxide and poss containing flame retardant photocured hybrid coatings. Prog. Org. Coat. 2017, 105, 37–47. [Google Scholar]
- Zhao, Y.; Jizhi, Z.; Shifeng, Z.; Qiang, G.; Jianzhang, L.; Wei, Z. Synthesis and Mechanism of Metal-Mediated Polymerization of Phenolic Resins. Polymers 2016, 8, 159. [Google Scholar]
- Pilato, L. Phenolic resins: 100Years and still going strong. React. Funct. Polym. 2013, 73, 270–277. [Google Scholar] [CrossRef]
- Jun-ichi, O.; Wataru, O.; Asao, O. A TG-MS study of poly(vinyl butyral)/phenol-formaldehyde resin blend fiber. Carbon 2000, 38, 1515–1519. [Google Scholar]
- Wang, J.; Jiang, H.; Jiang, N. Study on the pyrolysis of phenol-formaldehyde (PF) resin and modified PF resin. Thermochim. Acta 2009, 496, 136–142. [Google Scholar]
- Costa, L.; Di Montelera, L.R.; Camino, G.; Weil, E.D.; Pearce, E.M. Structure-charring relationship in phenol-formaldehyde type resins. Polym. Degrad. Stab. 1997, 56, 23–35. [Google Scholar] [CrossRef]
- Zahra, E.; Farshad, Y.; Mir, A.M. Thermal and mechanical properties of phenolic-based composites reinforced by carbon fibres and multiwall carbon nanotubes. Compos. Part. A 2015, 72, 22–31. [Google Scholar]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The pyrolysis mechanism of phe-nol formaldehyde resin. Polym. Degrad. Stab. 2012, 97, 1527–1533. [Google Scholar]
- Liu, C.L.; Dong, W.S.; Song, J.R.; Liu, L. Evolution of microstructure and properties of phenolic fibers during carbonization. Mater. Sci. Eng. A 2007, 459, 347–354. [Google Scholar]
- Nair, C.R.; Bindu, R.L.; Ninan, K.N. Thermal characteristics of addition-cure phenolic resins. Polym. Degrad. Stab. 2001, 73, 251–257. [Google Scholar] [CrossRef]
- Wang, S.; Xing, X.; Wang, W.; Jing, X. Influence of poly (dihydroxybiphenyl borate) on the curing behaviour and thermal pyrolysis mechanism of phenolic resin. Polym. Degrad. Stab. 2017, 144, 378–391. [Google Scholar]
- Liu, Y.; Jing, X. Pyrolysis and structure of hyperbranched polyborate modified phenolic resins. Carbon 2007, 45, 1965–1971. [Google Scholar]
- Xu, F.; Zhu, S.; Ma, Z.; Liu, Y.; Li, H.; Hu, J. Improved interfacial strength and ablation resistance of carbon fabric reinforced phenolic composites modified with functionalized ZrSiO4 sol. Mater. Des. 2020, 191, 108623. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Ding, J.; Wang, B.; Zhuang, Y.; Huang, Z. Synthesis and Thermal Degradation Study of Polyhedral Oligomeric Silsesquioxane (POSS) Modified Phenolic Resin. Polymers 2021, 13, 1182. https://doi.org/10.3390/polym13081182
Wang D, Ding J, Wang B, Zhuang Y, Huang Z. Synthesis and Thermal Degradation Study of Polyhedral Oligomeric Silsesquioxane (POSS) Modified Phenolic Resin. Polymers. 2021; 13(8):1182. https://doi.org/10.3390/polym13081182
Chicago/Turabian StyleWang, Degang, Jie Ding, Bing Wang, Yingluo Zhuang, and Zhixiong Huang. 2021. "Synthesis and Thermal Degradation Study of Polyhedral Oligomeric Silsesquioxane (POSS) Modified Phenolic Resin" Polymers 13, no. 8: 1182. https://doi.org/10.3390/polym13081182