Review on Spinning of Biopolymer Fibers from Starch
Abstract
:1. Introduction
2. Starch as a Source of Fiber
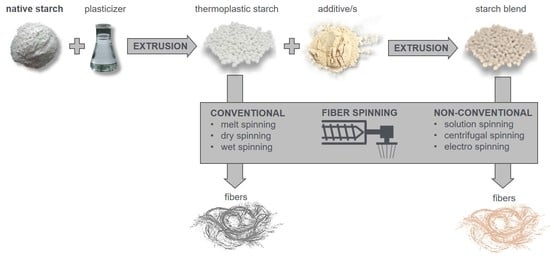
2.1. Fiber Spinning from Thermoplastic Starch Compositions
2.2. Spinning Non-Thermoplastic Starch Compositions
3. Possible Spinning Techniques for Starch Biopolymer
3.1. Conventional Fiber Spinning Methods
3.2. Modern Techniques Used for Starch Fiber Spinning
3.2.1. Electrospinning of Fibers from Starch
3.2.2. Electrospun Fibers from Starch/Polymer Blends
3.2.3. Electro Wet Spinning
3.2.4. Centrifugal Spinning
3.2.5. Solution Blow Spinning
3.3. Comparison of the Different Fiber Spinning Methods
4. Application of Bio-Based Materials
Application of Starch Biopolymers
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trade and Development Report 2019: Financing a Global Green New Deal; United Nations Conference on Trade and Development: Geneva, Switzerland, 2019.
- Sadasivuni, K.K.; Saha, P.; Adhikari, J.; Deshmukh, K.; Ahamed, M.B.; Cabibihan, J.J. Recent advances in mechanical properties of biopolymer composites: A review. Polym. Compos. 2020, 41, 32–59. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha-Ray, S. A review on biopolymer-based fibers via electrospinning and solution blowing and their applications. Fibers 2018, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Hemamalini, T.; Giri Dev, V.R. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Belén, M.; Encalada, K.; Proaño, E. An overview of starch-based biopolymers and their biodegradability Una revisión sobre biopolímeros con base en almidón y su biodegradabilidad. Cienc. E Ing. 2018, 39, 245–258. [Google Scholar]
- Fink, J.K. Carbohydrate Related Polymers. Chem. Bio-Based Polym. 2014, 137–170. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Deri, F. Thermoplastic starch blends: A review of recent works. Polym. Sci. Ser. A 2012, 54, 165–176. [Google Scholar] [CrossRef]
- Babu, R.P.; Connor, K.O.; Seeram, R. Current progress on bio-based polymers and their future trends. Progress Biomater. 2013, 2, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Azwar, E.; Hakkarainen, M. Tuning the Mechanical Properties of Tapioca Starch by Plasticizers, Inorganic Fillers and Agrowaste-Based Fillers. Int. Sch. Res. Not. 2012, 201. [Google Scholar] [CrossRef] [Green Version]
- Article, R.; Neelam, K.; Vijay, S.; Lalit, S. Various Techniques for modification of starch and the application of its derivatives. Int. Res. J. Pharm. 2012, 3, 25–31. [Google Scholar]
- Marjadi, D.; Dharaiya, N.A. Bioplastic: A better alternative for sustainable future. Everyman Sci. 2010, 15, 90–92. [Google Scholar]
- Fitzgerald, A.; Proud, W.; Kandemir, A.; Murphy, R.J.; Jesson, D.A.; Trask, R.S.; Hamerton, I.; Longana, M.L. A Life Cycle Engineering Perspective on Biocomposites as a Solution for a Sustainable Recovery. Sustainability 2021, 13, 1160. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R.; Bhosale, R. Fibers spun from polysaccharides. In Handbook of Carbohydrate Polymers: Development, Properties and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 1–43. ISBN 9781608763672. [Google Scholar]
- Ashraf, R.; Sofi, H.S.; Malik, A.; Beigh, M.A.; Hamid, R.; Sheikh, F.A. Recent Trends in the Fabrication of Starch Nanofibers: Electrospinning and Non-electrospinning Routes and Their Applications in Biotechnology. Appl. Biochem. Biotechnol. 2019, 187, 47–74. [Google Scholar] [CrossRef]
- Antonio, J.F. Starch: Major Sources, Properties and Applications as Thermoplastic Materials. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Curvelo, A.A.S.; De Carvalho, A.J.F.; Agnelli, J.A.M. Thermoplastic starch-cellulosic fibers composites: Preliminary results. Carbohydr. Polym. 2001, 45, 183–188. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R. Fabrication of pure starch fibers by electrospinning. Food Hydrocoll. 2014, 36, 20–25. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R. Patents on Fiber Spinning from Starches. Recent Pat. Food Nutr. Agric. 2012, 4, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Zussman, E.; Lancuski, A. High-amylose starch- formate electrospun fibers. WO 2016/132370, 25 August 2016. [Google Scholar]
- Bailey, V.A.; Mackey, L.N.; Trokhan, P.D. Starch fiber. US7704328B2, 27 April 2010. [Google Scholar]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Chadehumbe, C. Tensile Properties of Thermoplastic Starch and Its Blends With Polyvinyl Butyral and Polyamides. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, August 2006. [Google Scholar]
- Bailey, V.A.; Mackey, L.N.; Trokhan, P.D. Melt processable starch compositions. US7666261B2, 23 February 2010. [Google Scholar]
- Huneault, M.; Li, H. Process of Producing Thermoplastic Starch/Polymer Blends. US9045625B2, 2 June 2015. [Google Scholar]
- Guzmán, M.; Murillo, E.A. Structural, thermal, rheological, morphological and mechanical properties of thermoplastic starch obtained by using hyperbranched polyester polyol as plasticizing agent. DYNA 2018, 85, 178–186. [Google Scholar] [CrossRef]
- Tanaka, H.; Miyahara, Y.; Kasetani, S.; Esaki, K.; Nishimura, S.; Inoue, T. Biodegradable nonwoven fabrics and method of manufacturing same. US5614298A, 25 March 1997. [Google Scholar]
- Fonseca, L.M.; de Oliveira, J.P.; de Oliveira, P.D.; da Rosa Zavareze, E.; Dias, A.R.G.; Lim, L.T. Electrospinning of native and anionic corn starch fibers with different amylose contents. Food Res. Int. 2019, 116, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Tuzlakoglu, K.; Pashkuleva, I.; Rodrigues, M.T.; Gomes, M.E. A new route to produce starch-based fiber mesh scaffolds by wet spinning and subsequent surface modification as a way to improve cell attachment and proliferation. J. Biomed. Mater. Res. A 2009, 92, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Bond, E.B.; Wheeler, D.S.; Arora, K.A. High elongation splittable multicomponent fibers comprising starch and polymers. US6743506B2, 1 June 2004. [Google Scholar]
- Bond, E.B.; Autran, J.-P.M.; Mackey, L.N.; Noda, I.; O´Donnell, H.J. Fibers comprising starch and biodegradable polymers. US6946506B2, 20 September 2005. [Google Scholar]
- Lorcks, J.; Pommeranz, W.; Schmidt, H. Biodegradable fibers manufactured from thermoplastic starch and textile products and other articles manufactured from such fibers. US6218321B1, 17 April 2001. [Google Scholar]
- Nakajima, Y.; Taniguchi, M. Biodegradable fiber and non-woven fabric. US6045908A, 4 April 2000. [Google Scholar]
- Eden, J.; Trksak, M. Process for spinning starch fibers. US4853168A, 1 August 1989. [Google Scholar]
- James, M.D.; Mackey, L.N.; Ensign, D.E.; Aydore, S. Process for making non-thermoplastic starch fibers. US6811740B2, 2 November 2004. [Google Scholar]
- James, M.D.; Mackey, L.N.; Ensign, D.E.; Aydore, S. Process for making non-thermoplastic starch fibers. US7276201B2, 2 October 2007. [Google Scholar]
- Bastioli, C.; Casale, B.; Zanardi, G. Device and process for the production of fibrous starch materials. WO1994009190, 28 April 1994. [Google Scholar]
- William, A.C.; Efrén, M.P.; Yesid, G.P.E.; Ricardo, V.G. Comparative Study of Starch Fibers Obtained by Electro-spinning of Indigenous, Commercial and Cationic Potato Starch. J. Nat. Fibers 2020, 17, 809–819. [Google Scholar] [CrossRef]
- Ebner von Eschenbach, J. Electro-spinning process for making starch filaments for flexible structure. EP1217107A1, 26 June 2002. [Google Scholar]
- Nayak, R.; Padhye, R. Nano Fibres by Electro spinning: Properties and Applications. J. Text. Eng. Fash. Technol. 2017, 2, 486–497. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Ziegler, G.R. Formation of starch-guest inclusion complexes in electrospun starch fibers. Food Hydrocoll. 2014, 38, 211–219. [Google Scholar] [CrossRef]
- Cárdenas, W.; Gómez-Pachon, E.Y.; Muñoz, E.; Vera-Graziano, R. Preparation of potato starch microfibers obtained by electro wet spinning. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 138. [Google Scholar] [CrossRef]
- Jaiturong, P.; Sutjarittangtham, K.; Eitsayeam, S.; Sirithunyalug, J. Preparation of glutinous rice starch nanofibers by electrospinning. Adv. Mater. Res. 2012, 506, 230–233. [Google Scholar] [CrossRef]
- Jaiturong, P.; Intatha, U.; Eitssayeam, S.; Sirithunyalug, J. Fabrication of Natural Tapioca Starch Fibers by a Modified Electrospinning Technique. Chiang Mai J. Sci. 2014, 41, 213–223. [Google Scholar]
- Komur, B.; Bayrak, F.; Ekren, N.; Eroglu, M.S.; Oktar, F.N.; Sinirlioglu, Z.A.; Yucel, S.; Guler, O.; Gunduz, O. Starch/PCL composite nanofibers by co-axial electrospinning technique for biomedical applications. Biomed. Eng. Online 2017, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, H.; Yang, B. Centrifugally spun starch-based fibers from amylopectin rich starches. Carbohydr. Polym. 2016, 137, 459–465. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Bastiaansen, C.W.M.; Peijs, T.; Rogalski, J.J.; Bastiaansen, C.W.M.; Peijs, T. Rotary jet spinning review—A potential high yield future for polymer nanofibers. Nanocomposites 2017, 3, 97–121. [Google Scholar] [CrossRef] [Green Version]
- Vadas, D.; Kmetykó, D.; Marosi, G.; Bocz, K. Application of melt-blown Poly(lactic acid) fibres in self-reinforced composites. Polymer (Basel) 2018, 10, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancuški, A.; Vasilyev, G.; Putaux, J.L.; Zussman, E. Rheological Properties and Electrospinnability of High-Amylose Starch in Formic Acid. Biomacromolecules 2015, 16, 2529–2536. [Google Scholar] [CrossRef]
- Fonseca, L.M.; da Silva, F.T.; Antunes, M.D.; Mello el Halal, S.L.; Lim, L.T.; Dias, A.R.G. Aging Time of Soluble Potato Starch Solutions for Ultrafine Fibers Formation by Electrospinning. Starch Staerke 2019, 71, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Electrospun starch nanofibers: Recent advances, challenges, and strategies for potential pharmaceutical applications. J. Control. Release 2017, 252, 95–107. [Google Scholar] [CrossRef]
- Šukyte, J.; Adomavičiute, E.; Milašius, R. Investigation of the possibility of forming nanofibres with potato starch. Fibres Text. East. Eur. 2010, 82, 24–27. [Google Scholar]
- Wang, H.; Wang, W.; Jiang, S.; Jiang, S.; Zhai, L.; Jiang, Q. Poly(vinyl alcohol)/oxidized starch fibres via electrospinning technique: Fabrication and characterization. Iran. Polym. J. Engl. Ed. 2011, 20, 551–558. [Google Scholar]
- Jukola, H.; Nikkola, L.; Ashammakhi, N. Electrospun Starch-Polycaprolactone Nanofiber-Based Constructs for Tissue Engineering. AIP Conf. Proc. 2008, 973, 971–975. [Google Scholar] [CrossRef]
- Sunthornvarabhas, J.; Chatakanonda, P.; Piyachomkwan, K.; Sriroth, K. Electrospun polylactic acid and cassava starch fiber by conjugated solvent technique. Mater. Lett. 2011, 65, 985–987. [Google Scholar] [CrossRef]
- Adomavičiute, E.; Milašius, R.; Žemaitaitis, A.; Bendoraitiene, J.; Leskovšek, M.; Demšar, A. Methods of forming nanofibres from bicomponent PVA/Cationic starch solution. Fibres Text. East. Eur. 2009, 74, 29–33. [Google Scholar]
- Šateike, J.; Milašius, R. Influence of Modified Cationic Starch in a Mixed Poly(Vinyl Alcohol)/Cationic Starch Solution on the Electrospinning Process and Web Structure. Autex Res. J. 2020, 20, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Kong, L.; Ziegler, G.R. Fabrication of starch—Nanocellulose composite fibers by electrospinning. Food Hydrocoll. 2019, 90, 90–98. [Google Scholar] [CrossRef]
- Lancuˇ, A.; Ammar, A.A.; Avrahami, R.; Vilensky, R.; Vasilyev, G. Design of Starch-Formate Compound Fibers as Encapsulation Platform for Biotherapeutics Design of starch-formate compound fibers as encapsulation platform for biotherapeutics. Carbohydr. Polym. 2016, 158, 68–76. [Google Scholar] [CrossRef]
- Garalde, R.A.; Thipmanee, R.; Jariyasakoolroj, P.; Sane, A. The e ff ects of blend ratio and storage time on thermoplastic starch / poly (butylene adipate- co -terephthalate ) fi lms. Heliyon 2019, 5, e01251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Hou, T.; Lu, Y.; Yang, B. A method for controlling the surface morphology of centrifugally spun starch-based fibers. J. Appl. Polym. Sci. 2018, 135, 45810. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H. A review on tensile and morphological properties of poly (lactic acid) (PLA)/ thermoplastic starch (TPS) blends. Polym. Technol. Mater. 2019, 58, 1945–1964. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Glenn, G.M.; Klamczynski, A.P.; Orts, W.J.; Mattoso, L.H.C. Solution Blow Spinning: A New Method to Produce Micro- and Nanofibers from Solution Blow Spinningg: A New Method to Produce Micro- and Nanofibers from Polymer Solutions. J. Appl. Polym. Sci. 2009, 113, 2322–2330. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yeom, B.Y.; Wilkie, A.; Pourdeyhimi, B.; Khan, S.A. Fabrication of nanofiber meltblown membranes and their filtration properties. J. Memb. Sci. 2013, 427, 336–344. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Mohan, S.; Oluwafemi, O.S.; Kalarikkal, N.; Thomas, S.; Songca, S.P. Biopolymers—Application in Nanoscience and Nanotechnology. Recent Adv. Biopolym. 2016, 1, 47–66. [Google Scholar]
- Neves, A.C.C.; Rohen, L.A.; Mantovani, D.P.; Carvalho, J.P.R.G.; Vieira, C.M.F.; Lopes, F.P.D.; Simonassi, N.T.; Luz, F.S.D.; Monteiro, S.N. Comparative mechanical properties between biocomposites of Epoxy and polyester matrices reinforced by hemp fiber. J. Mater. Res. Technol. 2020, 9, 1296–1304. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Synthesis and Applications of Biopolymer Composites. Int. J. Mol. Sci. 2019, 20, 2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilla, S. Engineering Applications of Bioplastics and Biocomposites—An Overview. In Handbook of Bioplastics and Biocomposites Engineering Applications; Pilla, S., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC: Salem, MA, USA, 2011. [Google Scholar]
- Vahabi, H.; Rastin, H.; Movahedifar, E.; Antoun, K.; Brosse, N.; Saeb, M.R. Flame Retardancy of Bio-Based Polyurethanes: Opportunities and Challenges. Polymers 2020, 12, 1234. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Singhvi, M.; Gokhale, D. Biomass to biodegradable polymer (PLA). RSC Adv. 2013, 3, 13558–13568. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- RameshKumar, S.; Shaiju, P.; O’Connor, K.E. Bio-based and biodegradable polymers—Tate-of-the-art, challenges and emerging trends. Curr. Opin. Green Sustain. Chem. 2020, 21, 75–81. [Google Scholar] [CrossRef]
- Kumar, R.; Ha, S.K.; Verma, K.; Tiwari, S.K. Recent progress in selected bio-nanomaterials and their engineering applications: An overview. J. Sci. Adv. Mater. Devices 2018, 3, 263–288. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based Biodegradable Materials: Challenges and Opportunities. Adv. Ind. Eng. Polym. Res. 2019, 3, 8–18. [Google Scholar] [CrossRef]
- Johansson, C.; Bras, J.; Mondragon, I.; Nechita, P.; Plackett, D.; Šimon, P.; Svetec, D.G.; Virtanen, S.; Baschetti, M.G.; Breen, C.; et al. Renewable fibers and bio-based materials for packaging applications—A review of recent developments. BioResources 2012, 7, 2506–2552. [Google Scholar] [CrossRef] [Green Version]
- Pandey, J.K.; Pratheep Kumar, A.; Misra, M.; Mohanty, A.K.; Drzal, L.T.; Singh, R.P. Recent advances in biodegradable nanocomposites. J. Nanosci. Nanotechnol. 2005, 5, 497–526. [Google Scholar] [CrossRef] [PubMed]
- Fahma, F.; Sunarti, T.C.; Indriyani, S.M.; Lisdayana, N. Thermoplastic Cassava Starch-PVA Composite Films with Cellulose Nanofibers from Oil Palm Empty Fruit Bunches as Reinforcement Agent. Int. J. Polym. Sci. 2017, 2017, 2745721. [Google Scholar] [CrossRef] [Green Version]
- Muhammadi, S.; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar] [CrossRef] [Green Version]
- Bugnicourt, E.; Schmid, M.; Nerney, O.M.; Wildner, J.; Smykala, L.; Lazzeri, A.; Cinelli, P. Processing and validation of whey-protein-coated films and laminates at semi-industrial scale as novel recyclable food packaging materials with excellent barrier properties. Adv. Mater. Sci. Eng. 2013, 2013, 496207. [Google Scholar] [CrossRef] [Green Version]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar]
- Xu, J.; Guo, B.-H. Microbial Succinic Acid, Its Polymer Poly(Butylene Succinate), and Applications. In Plastics from Bacteria; Chen, G.G.-Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Seggiani, M.; Gigante, V.; Cinelli, P.; Coltelli, M.B.; Sandroni, M.; Anguillesi, I.; Lazzeri, A. Processing and mechanical performances of Poly(Butylene Succinate–co–Adipate) (PBSA) and raw hydrolyzed collagen (HC) thermoplastic blends. Polym. Test. 2019, 77, 105900. [Google Scholar] [CrossRef]
- Kahve, H.I.; Ardic, M. Lipid-Based Edible Films. J. Sci. Eng. Res. 2017, 4, 86–92. [Google Scholar]
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced material applications of starch and its derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar] [CrossRef]
- Waheed, Z.; Dong, Y. Recent Advances and Perspectives on Starch Nanocomposites for Packaging Applications. J. Mater. Sci. 2018, 53, 15319–15339. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers 2019, 7, 32. [Google Scholar]
- Boudjema, H.L.; Bendaikha, H. Composite materials derived from biodegradable starch polymer and Atriplex halimus fibers. e-Polymers 2015, 15, 419–426. [Google Scholar] [CrossRef]
- Lv, H.; Cui, S.; Zhang, H.; Pei, X.; Gao, Z.; Hu, J.; Zhou, Y.; Liu, Y. Crosslinked starch nanofibers with high mechanical strength and excellent water resistance for biomedical applications. Biomed. Mater. 2020, 15, 025007. [Google Scholar] [PubMed]
- Cerqueira, J.C.; Penha, S.; Oliveira, R.S.; Lefol, L.; Guarieiro, N.; Melo, S.; Viana, J.D.; Aparecida, B.; Machado, S. Production of biodegradable starch nanocomposites using cellulose nanocrystals extracted from coconut fibers. Polímeros 2017, 27, 320–329. [Google Scholar]
- Yu, M.; Wang, Y. Starch-based nanoparticles: Stimuli responsiveness, toxicity, and interactions with food components. Comprehens. Rev. Food Sci. Food Saf. 2021, 20, 1075–1100. [Google Scholar] [CrossRef]
- Fazeli, M.; Keley, M.; Biazar, E. Preparation and characterization of starch-based composite films reinforced by cellulose nanofibers. Int. J. Biol. Macromol. 2018, 116, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.; Fu, J.; Wallen, S.L.; Hill, C.; Carolina, N. Completely “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Ceram. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef] [PubMed]
- Gregorová, E.; Pabst, W.; Smith, D.S.; Zivcová, Z. Thermal conductivity of porous alumina ceramics prepared using starch as a pore-forming agent. J. Eur. Ceram. Soc. 2009, 29, 347–353. [Google Scholar] [CrossRef]
- Mansourighasri, A.; Muhamad, N.; Sulong, A.B. Processing titanium foams using tapioca starch as a space holder. J. Mater. Process. Tech. 2012, 212, 83–89. [Google Scholar] [CrossRef]
- Alves, H.M.; Tarì, G.; Fonseca, A.T.; Ferreira, J.M.F. Processing of Porous Cordierite Bodies by starch consolidation. Mater. Res. Bull. 1998, 33, 1439–1448. [Google Scholar] [CrossRef]
- Davis, J.B.; Kristoffersson, A.; Carlstro, E.; Clegg, W.J. Fabrication and Crack Deflection in Ceramic Laminates with Porous Interlayers. J. Am. Ceram. Soc. 2000, 74, 2369–2374. [Google Scholar] [CrossRef]
- He, F.; Zhao, D. Preparation and Characterization of a New Class of Starch-Stabilized Bimetallic Nanoparticles for Degradation of Chlorinated Hydrocarbons in Water. Environ. Sci.Technol. 2005, 39, 3314–3320. [Google Scholar] [CrossRef]
- Sami, A.J.; Khalid, M.; Iqbal, S.; Afzal, M.; Shakoori, A.R. Synthesis and Application of Chitosan- Starch Based Nanocomposite in Wastewater Treatment for the Removal of Anionic Commercial Dyes. Pak. J. Zool. 2017, 49, 21–26. [Google Scholar] [CrossRef]
- Woranuch, S.; Pangon, A.; Puagsuntia, K.; Subjalearndee, N. Starch-based and multi-purpose nanofibrous membrane for high efficiency nanofiltration. RSC Adv. 2017, 7, 35368–35375. [Google Scholar] [CrossRef] [Green Version]
- Mirab, F.; Eslamian, M.; Bagheri, R. Fabrication and characterization of a starch-based nanocomposite scaffold with highly porous and gradient structure for bone tissue engineering. Biomed. Phys. Eng. Express 2018, 4, 055021. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Keynton, R.S. Fabrication and characterization of biopolymer fibers for 3D oriented microvascular structures. J. MicroMech. MicroEng. 2019, 29, 083003. [Google Scholar] [CrossRef]
- Odeku, O.A.; Akinwande, B.L. Effect of the mode of incorporation on the disintegrant properties of acid modified water and white yam starches. Saudi Pharm. J. 2012, 20, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odeku, O.A.; Schmid, W.; Picker-freyer, K.M. Material and tablet properties of pregelatinized (thermally modified ) Dioscorea starches. Eur. J. Pharm. Biopharm. 2008, 70, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Santander-ortega, M.J.; Stauner, T.; Loretz, B.; Ortega-vinuesa, J.L.; Bastos-gonzález, D.; Wenz, G. Nanoparticles made from novel starch derivatives for transdermal drug delivery. J. Control. Release 2010, 141, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, A.; Mariadoss, A.; Saravanakumar, K.; Sathiyaseelan, A. Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol. 2020, 164, 2073–2084. [Google Scholar] [CrossRef]
- Ju, B.; Yan, D.; Zhang, S. Micelles self-assembled from thermoresponsive 2-hydroxy-3-butoxypropyl starches for drug delivery. Carbohydr. Polym. 2012, 87, 1404–1409. [Google Scholar] [CrossRef]
- Odeku, O.A. Potentials of tropical starches as pharmaceutical excipients: A review. Starch Stärke 2013, 65, 89–106. [Google Scholar] [CrossRef]
- Odeku, O.A.; Picker-freyer, K.M. Characterization of acid modified Dioscorea starches as direct compression excipient. Pharm. Dev. Technol. 2009, 14, 259–270. [Google Scholar] [CrossRef]
- Guan, Y.; Qian, L.; Xiao, H.; Zheng, A. Preparation of novel antimicrobial-modified starch and its adsorption on cellulose fibers: Part I. Optimization of synthetic conditions and antimicrobial activities. Cellulose 2008, 15, 609–618. [Google Scholar] [CrossRef]
- Khalid, S.; Yu, L.; Feng, M.; Meng, L.; Bai, Y.; Ali, A.; Liu, H.; Chen, L. Development and characterization of biodegradable antimicrobial packaging fi lms based on polycaprolactone, starch and pomegranate rind hybrids. Food Packag. Shelf Life 2018, 18, 71–79. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Nazrin, A.; Sherwani, S.F.K. Antimicrobial Activities of Starch-Based Biopolymers and Biocomposites Incorporated with Plant Essential Oils: A Review. Polymers 2020, 12, 2403. [Google Scholar] [CrossRef] [PubMed]
- Pelissari, F.M.; Grossmann, M.V.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, Mechanical, and Barrier Properties of Cassava Starch—Chitosan Films Incorporated with Oregano Essential Oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Min, J.; Chen, Y.; Zhao, G. Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll. 2010, 24, 285–290. [Google Scholar] [CrossRef]
- Ziaee, Z.; Qian, L.; Guan, Y.; Fatehi, P.; Xiao, H. Antimicrobial/Antimold Polymer-Grafted Starches for Recycled Cellulose Fibers. J. Biomater. Sci. 2012, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.R.; Netravali, A.N. Self-healing starch-based ‘green’ thermoset resin. Polymer 2017. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Xia, K.; Xu, Z.; Lou, H.; Zhang, H. Starch Paper-Based Triboelectric Nanogenerator for Human Perspiration Sensing. Nanoscale Res. Lett. 2018, 13, 365. [Google Scholar] [CrossRef]
- Jeong, H.; Baek, S.; Han, S.; Jang, H.; Kim, S.H. Novel Eco-Friendly Starch Paper for Use in Flexible, Transparent, and Disposable Organic Electronics. Adv. Funct. Mater. 2018, 28, 1704433. [Google Scholar] [CrossRef]
- Cyprych, K.; Sznitko, L.; Mysliwiec, J. Starch: Application of biopolymer in random lasing. Org. Electron. 2014, 15, 2218–2222. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, P.; Kuang, Y.; Liu, Y.; Lin, D.; Peng, C. Durable superhydrophobic paper enabled by surface sizing of starch-based composite films. Appl. Surf. Sci. 2017, 409, 45–51. [Google Scholar] [CrossRef]
- Sun, B.; Xie, G.; Jiang, Y.; Li, X. Comparative CO2-Sensing Characteristic Studies of PEI and PEI / Starch Thin Film Sensors. Energy Procedia 2011, 12, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Khachatryan, G.C.; Khachatryan, K. Starch based nanocomposites as sensors for heavy metals—Detection of Cu2+ and Pb2+ ions. Int. AgroPhys. 2019, 33. [Google Scholar] [CrossRef]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and characterization of intelligent starch/PVA films for simultaneous colorimetric indication and antimicrobial activity for food packaging applications. Carbohydr. Polym. 2016, 157, 642–849. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, P.; Alexandrova, T.; Karadjova, I. Application of Starch-Stabilized Silver Nanoparticles as a Colorimetric Sensor for Mercury (II) in 0.005 mol/L Nitric Acid. J. Chem. 2017, 2017, 6897960. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, L.M.; Radünz, M.; Santos, C.; Silva, F.T.; Camargo, T.M.; Bruni, G.P. Electrospun potato starch nanofibers for thyme essential oil encapsulation: Antioxidant activity and thermal resistance. J. Sci. Food Agric. 2020, 100, 4263–4271. [Google Scholar] [CrossRef] [PubMed]







| Title | Process Used | Patent and Year |
|---|---|---|
| Method of making amylostic filaments and fibers | Mixing and extrusion of amylostic solids, water and glycerine at elevated temperature | US3499074 (1970) |
| Starch-containing fibers, process for their production | Mixing and extrusion of composition | US5516815 (1996) |
| Biodegradable fiber and non-woven fabric | Forming the fibers and bonding by heating at moist condition | US6045908 (2000) |
| Biodegradable fibers manufactured from thermoplastic starch. | Extrusion of the composition above into filaments, drawing, winding, and knitting | US6218321 (2001) |
| High elongation multicomponent fibers comprising starch | Extrusion of substituted starch and polymer having sheath–core configuration | US6623854 (2003) |
| Melt processable starch compositions | Mixing and extrusion of the composition | US6709526 (2004) |
| High elongation splittable multicomponent fibers comprising starch | Mixing extrusion and highspeed spinning of composition | US6743506 (2004) |
| Multicomponent fibers comprising starch and polymers | Melt spinning of the composition | US6746766 (2004) |
| Bicomponent fibers comprising a thermoplastic polymer surrounding a starch-rich core | Melt extrusion of composition and fiber formation | US6783854 (2004a) |
| Compositions and processes for reducing water solubility of a starch component in a multicomponent fiber | Melt spinning of the composition | US6830810 (2004b) |
| Fibers comprising starch and biodegradable polymers | Compounding and extrusion of composition and then melt spinning | US6890872 (2005) |
| Electro-spinning process for making starch filaments for flexible structure | Electrospinning of a starch/polymer (PAM) composition | US7029620 (2006) |
| Rotary spinning processes for forming hydroxyl polymer-containing fibers | Rotary spinning method forstarch/PVA composition | US7655175 (2010) |
| Starch fiber | Melt blowing of the composition | US7704328 (2010b) |
| Multicomponent fibers comprising starch and polymers | ----------------- | US7851391 (2010) |
| Fibers comprising starch and a crosslinking agent | Melt blowing of the composition | US7938908 (2011) |
| Title | Process Used | Patent and Year |
|---|---|---|
| Water-insensitive starch fibers and a process for the production thereof | Wet spinning of native and modified starch into an ammonium sulfate bath | US4139699 (1979) |
| Process for spinning starch fibers | Wet spinning of native starch and ammonium sulfate aqueous dispersion into ammonium sulfate bath | US4853168 (1989) |
| Fiber from blend of cellulose acetate and starch acetate | --------------------- | US5446140 (1995) |
| Non-thermoplastic starch fibers and starch composition for making the same | ------------------- | US6723160 (2004a) |
| Non-thermoplastic starch fibers and starch composition for making the same | --------------------- | US6802895 (2004b) |
| Process for making non-thermoplastic starch fibers | Dry spinning of starch aqueous dispersion with high temperature attenuatingair flow | US6811740 (2004) |
| Non-thermoplastic starch fibers and starch composition for making the same | --------------------- | US7025821 (2006) |
| Process for making non-thermoplastic starch fibers | Dry spinning of starch aqueous dispersion with high temperature attenuating air flow | US7276201 (2007) |
| Method for making polymeric structures | Making fiber products from starch, PVOH, and a crosslinking agent (imidazolidinone) and further adding of cellulose fibers | US7744791 (2010) |
| Method for forming fibers | Mixing, extrusion, and drawing of fibers from starch/PVA composition | US7939010 (2011) |
| Electrospun Material | Solvent Used | Characteristics of Obtained Fibers | Year of Publication |
|---|---|---|---|
| High amylose pure starch | Dimethyl sulfoxide DMSO/water | Fibers with diameter in range of micrometer | 2014 |
| Pure maize starch with (70%) amylose content | 17% w aqueous formic acid solution | Diameter ranging from 80 to 300 nm | 2013 |
| High amylopectin corn starch and potato starch | 2% w/w caustic soda solution | Submicron average diameter | 2015 |
| Waxy rice starch | water | Multiple flaky layers, highly porous | 2016 |
| High amylose modified starch 70% acetic anhydride | Ionic liquid 1-ally-1–3 methylimidazolium chloride | Continuous smooth fibers Diameter from 10 to 100 nm | 2007 |
| Modified maize starch with high amylose and 70% acetic anhydride | Formic acid | Tensile strength depends on starch to acetate ratio, annealing time, and degree of substitution | 2009 |
| High amylose modified maize starch 50% acetic anhydride | Dimethyl sulfoxide DMSO | Ultrafine fibers | 2013 |
| High amylose modified starch 70% formic acid solution | 17% aqueous formic acid solution | Diameter ranging from 80 to 300 nm Elongation at break higher than native starch | 2015 |
| Acidified oxidized potato starch | DMSO | Smooth fibers at concentration up to 19% | 2012 |
| Natural tapiaco starch | Deionized water | Diameter from 1.3 to 14.5 µm | |
| Native and anionic corn starch | Formic acid | Fibers with diameter 70–264 nm | 2018 |
| Electrospun Materials | Solvent Used | Characteristics of Fibers Obtained | Year of Publication |
|---|---|---|---|
| Starch/PCL 30/70 wt.% | Acetic acid or chloroform | Diameter 130–180 nm Highly porous | 2005 |
| Starch/PCL 17% w/v | Chloroform/dimethyl formamide (DMF) (7:3) | Diameter from 400 nm to 1.4 µm | 2010 |
| Starch/PCL 30/70 wt.% | Chloroform/DMF (7:3) | Diameter approximately 400 nm fine morphology | 2008 |
| Starch/PCL 30/70 wt.% | Chloroform 40% w/v | Fiber diameter around 100 µm | 2010 |
| Potato starch (5 wt.%)/polyvinyl alcohol/PVA | Ethanol 5 wt.% | 2010 | |
| Soluble starch/PVA 1:1 or 1:3 | Water | Good morphology | 2014 |
| Oxidized starch (OS)/PVA | Water | Diameter affected by weight ratio of PAV/OS | 2011 |
| Cationic starch (CS)/PVA (3:1) | Water | 2012 | |
| Cationic starch (CS)/PVA | Ethanol/water | Thicker and stick nanofiber | 2009 |
| Starch/poly (lactide–co-glycolide) (PLGA) | Starch in DMSO and PLAGA in tetrahydrofuran (THF)/N | 2011 | |
| Cassava starch/PLA | PLA in dichloromethane, cassava starch in DMSO | Smooth fibers | 2011 |
| High amylose maize starch, cationic starch and Nanocellulose | Dimethyl sulfoxide and ethanol | Good strength | 2018 |
| Rice starch/PVA (25 wt.%) | Water and NaoH | Uniform fibers with diameter 36–151 nm | 2017 |
| Glutinous rice starch/PVA (2 w/v and 8 w/v) | Hot water | Smooth morphology with diameter 191–263 nm | 2017 |
| Starch formate/glycerol (17 wt.%) | Formic acid | Fibers with diameter 4.13 µm | 2017 |
| Corn starch/guar gum (3 wt.%) | Water | Fibers with diameter 95 nm | 2017 |
| Starch acetate (20 wt.%) | Formic acid/water (90:10 v/v) | Fibers with good tenacity and uniformity | 2009 |
| Carboxymethyl starch/PLA | Sodium dodecyl sulfate | Diameter 190–265 nm | 2019 |
| Fiber Spinning Methods | Advantages | Disadvantages |
|---|---|---|
| Melt spinning |
|
|
| Solution Spinning (dry and wet) |
|
|
| Melt electro-spinning |
|
|
| Solution electrospinning |
|
|
| Centrifugal spinning |
|
|
| Solution blow spinning |
|
|
| Template synthesis |
|
|
| Self-assembly |
|
|
| Melt blowing |
|
|
| Phase separation |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temesgen, S.; Rennert, M.; Tesfaye, T.; Nase, M. Review on Spinning of Biopolymer Fibers from Starch. Polymers 2021, 13, 1121. https://doi.org/10.3390/polym13071121
Temesgen S, Rennert M, Tesfaye T, Nase M. Review on Spinning of Biopolymer Fibers from Starch. Polymers. 2021; 13(7):1121. https://doi.org/10.3390/polym13071121
Chicago/Turabian StyleTemesgen, Selamu, Mirko Rennert, Tamrat Tesfaye, and Michael Nase. 2021. "Review on Spinning of Biopolymer Fibers from Starch" Polymers 13, no. 7: 1121. https://doi.org/10.3390/polym13071121