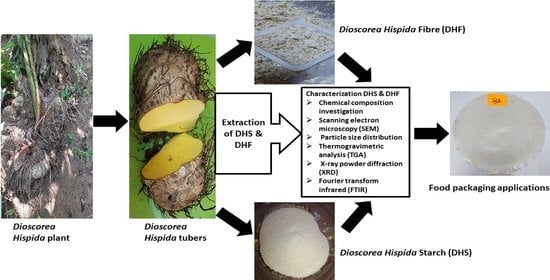
Extraction and Characterization of Potential Biodegradable Materials Based on Dioscorea hispida Tubers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Dioscorea hispida Starch and Fibres
2.3. Chemical Composition Analysis
2.4. Density
2.5. Moisture Content
2.6. Particle Size Distribution (PSD)
2.7. Scanning Electron Microscopy (SEM)
2.8. Thermogravimetric Analysis (TGA)
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. X-ray Diffraction (XRD)
3. Results
3.1. Chemical Composition
3.2. Thermogravimetric Analysis (TGA)
3.3. Morphology and Particle Size Analysis
3.4. FTIR Spectroscopy Analysis
3.5. X-ray Diffraction Analysis (XRD)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N.; Mohd Nurazzi, N.; Atiqah, A.; Ansari, M.N.M.; et al. Effect of sugar palm nanofibrillated celluloseconcentrations on morphological, mechanical andphysical properties of biodegradable films basedon agro-waste sugar palm (Arenga pinnata (Wurmb.) Merr) starch. J. Mater. Res. Technol. 2019, 8, 4819–4830. [Google Scholar] [CrossRef]
- Alsubari, S.; Zuhri, M.Y.M.; Sapuan, S.M.; Ishak, M.R.; Ilyas, R.A.; Asyraf, M.R.M. Potential of natural fiber reinforced polymer composites in sandwich structures: A review on its mechanical properties. Polymers 2021, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Sapuan, S.M.; Aulia, H.S.; Ilyas, R.A.; Atiqah, A.; Dele-Afolabi, T.T.; Nurazzi, M.N.; Supian, A.B.M.; Atikah, M.S.N. Mechanical properties of longitudinal basalt/woven-glass-fiber-reinforced unsaturated polyester-resin hybrid composites. Polymers 2020, 12, 2211. [Google Scholar] [CrossRef] [PubMed]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Characteristics of Eucheuma cottonii waste from East Malaysia: Physical, thermal and chemical composition. Eur. J. Phycol. 2017, 52, 200–207. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanofibrillated cellulose (Arenga pinnata (Wurmb.) Merr): Effect of cycles on their yield, physic-chemical, morphological and thermal behavior. Int. J. Biol. Macromol. 2019, 123, 379–388. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Effect of delignification on the physical, thermal, chemical, and structural properties of sugar palm fibre. BioResources 2017, 12, 8734–8754. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar] [CrossRef] [Green Version]
- Syafri, E.; Sudirman; Mashadi; Yulianti, E.; Deswita; Asrofi, M.; Abral, H.; Sapuan, S.M.; Ilyas, R.A.; Fudholi, A. Effect of sonication time on the thermal stability, moisture absorption, and biodegradation of water hyacinth (Eichhornia crassipes) nanocellulose-filled bengkuang (Pachyrhizus erosus) starch biocomposites. J. Mater. Res. Technol. 2019, 8, 6223–6231. [Google Scholar] [CrossRef]
- Asrofi, M.; Sapuan, S.M.; Ilyas, R.A.; Ramesh, M. Characteristic of composite bioplastics from tapioca starch and sugarcane bagasse fiber: Effect of time duration of ultrasonication (Bath-Type). Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Sanyang, M.; Sapuan, S.; Jawaid, M.; Ishak, M.; Sahari, J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atiqah, A.; Atikah, M.S.N.; Syafri, E.; Asrofi, M.; et al. Thermal, biodegradability and water barrier properties of bio-nanocomposites based on plasticised sugar palm starch and nanofibrillated celluloses from sugar palm fibres. J. Biobased Mater. Bioenergy 2020, 14, 234–248. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M. Biopolymers and biocomposites: Chemistry and technology. Curr. Anal. Chem. 2020, 16, 500–503. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M. The preparation methods and processing of natural fibre bio-polymer composites. Curr. Org. Synth. 2020, 16, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Jumaidin, R.; Khiruddin, M.A.A.; Asyul Sutan Saidi, Z.; Salit, M.S.; Ilyas, R.A. Effect of cogon grass fibre on the thermal, mechanical and biodegradation properties of thermoplastic cassava starch biocomposite. Int. J. Biol. Macromol. 2020, 146, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M. Sugar palm (Arenga pinnata [Wurmb.] Merr) starch films containing sugar palm nanofibrillated cellulose as reinforcement: Water barrier properties. Polym. Compos. 2019, 1–9. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Koloor, S.S.R.; Petrů, M. Micro- and nanocellulose in polymer composite materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Rozilah, A.; Jaafar, C.N.A.; Sapuan, S.M.; Zainol, I.; Ilyas, R.A. The effects of silver nanoparticles compositions on the mechanical, physiochemical, antibacterial, and morphology properties of sugar palm starch biocomposites for antibacterial coating. Polymers 2020, 12, 2605. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- Singh, G.; Jose, S.; Kaur, D.; Soun, B. Extraction and characterization of corn leaf fiber. J. Nat. Fibers 2020, 1–11. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Souza, N.F.; Ornaghi, H.L.; de Freitas, M.R. Comparative analysis of different chlorine-free extraction on oil palm mesocarp fiber. Ind. Crops Prod. 2020, 150, 112305. [Google Scholar] [CrossRef]
- Mali, S.; Debiagi, F.; Grossmann, M.V.E.; Yamashita, F. Starch, sugarcane bagasse fibre, and polyvinyl alcohol effects on extruded foam properties: A mixture design approach. Ind. Crops Prod. 2010. [Google Scholar] [CrossRef]
- Razali, N.; Salit, M.S.; Jawaid, M.; Ishak, M.R.; Lazim, Y. A study on chemical composition, physical, tensile, morphological, and thermal properties of roselle fibre: Effect of fibre maturity. BioResources 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Azammi, A.M.N.; Sapuan, S.M.; Ishak, M.R.; Sultan, M.T.H. Physical and damping properties of kenaf fi bre fi lled natural rubber/thermoplastic polyurethane composites. Def. Technol. 2020, 16, 29–34. [Google Scholar] [CrossRef]
- Chin, S.C.; Tee, K.F.; Tong, F.S.; Ong, H.R.; Gimbun, J. Thermal and mechanical properties of bamboo fiber reinforced composites. Mater. Today Commun. 2020, 23, 100876. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.M.; Ilyas, R.A. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2019, 106186. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Pratama, A.B.; Fajri, N.; Sapuan, S.M.; Ilyas, R.A. Transparent and antimicrobial cellulose film from ginger nanofiber. Food Hydrocoll. 2020, 98. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector. Polymers 2021, 13, 158. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Abdul Wahab, N.I. Corn starch (zea mays) biopolymer plastic reaction in combination with sorbitol and glycerol. Polymers 2021, 13, 242. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Recent developments in sugar palm (Arenga pinnata) based biocomposites and their potential industrial applications: A review. Renew. Sustain. Energy Rev. 2016, 54, 533–549. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Salit, M.S.; Mohamad Yusoff, M.Z.; Zainudin, E.S.; Ya, H.H. The crashworthiness performance of stacking sequence on filament wound hybrid composite energy absorption tube subjected to quasi-static compression load. J. Mater. Res. Technol. 2020, 9, 654–666. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Development and characterization of sugar palm nanocrystalline cellulose reinforced sugar palm starch bionanocomposites. Carbohydr. Polym. 2018, 202, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Hazrol, M.D.; Sapuan, S.M.; Ilyas, R.A.; Othman, M.L.; Sherwani, S.F.K. Electrical properties of sugar palm nanocrystalline cellulose reinforced sugar palm starch nanocomposites. Polimery 2020, 65, 363–370. [Google Scholar] [CrossRef]
- Aisyah, H.A.; Paridah, M.T.; Sapuan, S.M.; Ilyas, R.A.; Khalina, A.; Nurazzi, N.M.; Lee, S.H.; Lee, C.H. A Comprehensive review on advanced sustainable woven natural fibre polymer composites. Polymers 2021, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Rangappa, S.M.; Siengchin, S.; Dhakal, H.N. Green-composites: Ecofriendly and sustainability. Appl. Sci. Eng. Prog. 2020, 13. [Google Scholar] [CrossRef]
- Kumar, T.S.M.; Chandrasekar, M.; Senthilkumar, K.; Ilyas, R.A.; Sapuan, S.M.; Hariram, N.; Rajulu, A.V.; Rajini, N.; Siengchin, S. Characterization, thermal and antimicrobial properties of hybrid cellulose nanocomposite films with in-situ generated copper nanoparticles in Tamarindus indica Nut Powder. J. Polym. Environ. 2020, 1–10. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Khalina, A.; Sapuan, S.M.; Ilyas, R.A.; Rafiqah, S.A.; Hanafee, Z.M. Thermal properties of treated sugar palm yarn/glass fiber reinforced unsaturated polyester hybrid composites. J. Mater. Res. Technol. 2020, 9, 1606–1618. [Google Scholar] [CrossRef]
- Sari, N.H.; Pruncu, C.I.; Sapuan, S.M.; Ilyas, R.A.; Catur, A.D.; Suteja, S.; Sutaryono, Y.A.; Pullen, G. The effect of water immersion and fibre content on properties of corn husk fibres reinforced thermoset polyester composite. Polym. Test. 2020, 91, 106751. [Google Scholar] [CrossRef]
- Chandra Mohan, C.; Harini, K.; Vajiha Aafrin, B.; Lalitha priya, U.; Maria jenita, P.; Babuskin, S.; Karthikeyan, S.; Sudarshan, K.; Renuka, V.; Sukumar, M. Extraction and characterization of polysaccharides from tamarind seeds, rice mill residue, okra waste and sugarcane bagasse for its bio-thermoplastic properties. Carbohydr. Polym. 2018, 186, 394–401. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Wicklein, B.; Lo Dico, G.; Lazzara, G.; del Real, G.; Aranda, P.; Ruiz-Hitzky, E. Functional biohybrid materials based on halloysite, sepiolite and cellulose nanofibers for health applications. Dalt. Trans. 2020, 49, 3830–3840. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, R.A.; Sapuan, S.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Rafiqah, S.A.; Aisyah, H.A.; Nurazzi, N.M.; Norrrahim, M.N.F. Effect of hydrolysis time on the morphological, physical, chemical, and thermal behavior of sugar palm nanocrystalline cellulose (Arenga pinnata (Wurmb.) Merr). Text. Res. J. 2021, 91, 152–167. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N.; Ilyas, R.A. Woods and composites cantilever beam: A comprehensive review of experimental and numerical creep methodologies. J. Mater. Res. Technol. 2020, 9, 6759–6776. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Nazrin, A.; Sherwani, S.F.K.; Khalina, A. Antimicrobial activities of starch-based biopolymers and biocomposites incorporated with plant essential oils: A review. Polymers 2020, 12, 2403. [Google Scholar] [CrossRef] [PubMed]
- Azammi, A.M.N.; Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Atikah, M.S.N.; Asrofi, M.; Atiqah, A. Characterization studies of biopolymeric matrix and cellulose fibres based composites related to functionalized fibre-matrix interface. In Interfaces in Particle and Fibre Reinforced Composites- From Macro to Nano Scales; Woodhead Publishing: London, UK, 2019; pp. 1–68. ISBN 9780081026656. [Google Scholar]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications. Front. Chem. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Thermal, mechanical, and physical properties of seaweed/sugar palm fibre reinforced thermoplastic sugar palm starch/agar hybrid composites. Int. J. Biol. Macromol. 2017, 97, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and potential utilizations of starch from pineapple stem waste. Ind. Crops Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Mohd Izwan, S.; Sapuan, S.M.; Zuhri, M.Y.M.; Mohamed, A.R. Effects of benzoyl treatment on naoh treated sugar palm fiber: Tensile, thermal, and morphological properties. J. Mater. Res. Technol. 2020, 9, 5805–5814. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Tecante, A. Extraction and characterization of cellulose nanofibers from Rose stems (Rosa spp.). Carbohydr. Polym. 2019, 220, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chouw, N.; Jayaraman, K. Flax fibre and its composites—A review. Compos. Part B Eng. 2014, 56, 296–317. [Google Scholar] [CrossRef]
- Ganapathy, T.; Sathiskumar, R.; Senthamaraikannan, P.; Saravanakumar, S.S.; Khan, A. Characterization of raw and alkali treated new natural cellulosic fibres extracted from the aerial roots of banyan tree. Int. J. Biol. Macromol. 2019, 138, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Lazim, A.M.; Azman, I.; Yusoff, S.F.M.; Hassan, N.I.; Fazry, S.; Arip, M.N.M. Synthesis and characterization of Dioscorea hispida sp. tuber starch-polyacrylamide wood coating and its facile inhibitory towards Pycnoporus sanguineus and Coptotermes curvignathus. Prog. Org. Coatings 2016, 99, 182–190. [Google Scholar] [CrossRef]
- Hamid, Z.A.A.; Idris, M.H.M.; Arzami, N.A.A.B.; Ramle, S.F.M. Investigation on the chemical composition of Discorea hispida dennst (Ubi Gadong). AIP Conf. Proc. 2019, 2068. [Google Scholar] [CrossRef]
- Liu, Y.W.; Shang, H.F.; Wang, C.K.; Hsu, F.L.; Hou, W.C. Immunomodulatory activity of dioscorin, the storage protein of yam (Dioscorea alata cv. Tainong No. 1) tuber. Food Chem. Toxicol. 2007, 45, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Sasiwatpaisit, N.; Thitikornpong, W.; Palanuvej, C.; Ruangrungsi, N. Dioscorine content in Dioscorea hispida dried tubers in Thailand by TLC-densitometry and TLC image analysis. J. Chem. Pharm. Res. 2014, 6, 803–806. [Google Scholar]
- Kamaruddin, Z.H.; Sapuan, S.M.; Mohamed Yusoff, M.Z.; Jumaidin, R. Rapid detection and identification of dioscorine compounds in dioscorea hispida tuber plants by LC-ESI-MS. BioResources 2020, 15. [Google Scholar] [CrossRef]
- Nashriyah, M.; Salmah, M.Y.T.; Atiqah, N.; Indah, O.S.N.; MuhamadAzhar, A.W.; Munirah, S.; Nornasuha, Y.; Manaf, A.A. Ethnobotany and distribution of Dioscoreahispida dennst. (Dioscoreaceae) in Terengganu, Peninsular Malaysia. World Acad. Sci. Eng. Technol. 2012, 72, 1782–1785. [Google Scholar]
- Ashri, A.; Amalina, N.; Kamil, A.; Fazry, S.; Sairi, M.F.; Nazar, M.F.; Lazim, A.M. Modified Dioscorea hispida starch-based hydrogels and their in-vitro cytotoxicity study on small intestine cell line (FHS-74 Int). Int. J. Biol. Macromol. 2018, 107, 2412–2421. [Google Scholar] [CrossRef]
- Pascoal, A.M.; Di-Medeiros, M.C.B.; Batista, K.A.; Leles, M.I.G.; Lião, L.M.; Fernandes, K.F. Extraction and chemical characterization of starch from S. lycocarpum fruits. Carbohydr. Polym. 2013, 98, 1304–1310. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Preparation and characterization of cassava bagasse reinforced thermoplastic cassava starch. Fibers Polym. 2017, 18, 162–171. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Extraction, chemical composition, and characterization of potential lignocellulosic biomasses and polymers from corn plant parts. BioResources 2019. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Widiyanti, M.; Ratnawati, R.; Retnowati, D.S. Nutritional and functional properties changes during facultative submerged fermentation of gadung (Dioscorea hispida Dennst) tuber flour using Lactobacillus plantarum. Heliyon 2020, 6, e03631. [Google Scholar] [CrossRef] [PubMed]
- Mas, S. Study on starch granules of local varieties of Dioscorea hispida and dioscorea alata. J. Trop. Life Sci. 2016, 6, 47–52. [Google Scholar] [CrossRef]
- Susanto, T.; Affandy, R.; Katon, G. Rahmaniar, Thermal aging properties of natural rubber-styrene butadiene rubber composites filled with modified starch from Dioscorea Hispida Denst extract prepared by latex compounding method. AIP Conf. Proc. 2018, 2049. [Google Scholar] [CrossRef]
- Nugroho, L.H.; Estyaniyana, A. The potency of gadung (Dioscorea hispida Dennst.) tuber as a functional food: Toxicity, phytochemical content and starch characters. AIP Conf. Proc. 2018, 2002. [Google Scholar] [CrossRef]
- Mat, N.; Fatihah, H.N.N.; Sultan, U.; Abidin, Z.; Fatihah, N.; Nudin, H.; Arumugam, N. The distribution of dioscorea hispida Dennst. Germplasm in Terengganu and phylogenetic relationships of Dioscorea spp. using Internal Transcribed Spacer (ITS). J. Agrobiotechnol. 2014, 5, 31–47. [Google Scholar]
- Alam, P.N.; Mukhlishien; Husin, H.; Asnawi, T.M.; Santia; Yustira, A. The utilization of gadung (dioscorea hispida dennst) starch for edible coating making and its tomato packaging. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543. [Google Scholar] [CrossRef] [Green Version]
- Baraheng, S.; Karrila, T. Chemical and functional properties of durian (Durio zibethinus Murr.) seed flour and starch. Food Biosci. 2019, 30, 100412. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanocrystalline cellulose reinforced sugar palm starch composite: Degradation and water-barrier properties. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012006. [Google Scholar] [CrossRef] [Green Version]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Cassava: Its polymer, fiber, composite, and application. Polym. Compos. 2017, 38, 555–570. [Google Scholar] [CrossRef] [Green Version]
- Andrés, C.; Gordillo, S.; Valencia, G.A.; Antonio, R.; Zapata, V. Physicochemical characterization of arrowroot starch (Maranta arundinacea Linn) and glycerol/arrowroot starch membranes. Int. J. Food Eng. 2014, 10, 727–735. [Google Scholar] [CrossRef]
- Swinkels, J.J.M. Composition and properties of commercial native Starches. Starch Stärke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- Polycarp, D.; Afoakwa, E.O.; Budu, A.S.; Otoo, E. Characterization of chemical composition and anti-nutritional factors in seven species within the Ghanaian yam (Dioscorea) germplasm. Int. Food Res. J. 2012, 19, 985–992. [Google Scholar]
- Alotaibi, M.D.; Alshammari, B.A.; Saba, N.; Alothman, O.Y.; Sanjay, M.R.; Almutairi, Z.; Jawaid, M. Characterization of natural fiber obtained from different parts of date palm tree (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2019, 135, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Abdan, K.; Ibrahim, N.A. Preparation and characterization of fire retardant nano-filler from oil palm empty fruit bunch fibers. BioResources 2015, 10, 4530–4543. [Google Scholar] [CrossRef]
- Azlin, M.N.M.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Ilyas, R.A. Natural polylactic acid-based fiber composites: A review. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Al-Oqla, F.M., Sapuan, S.M., Eds.; Elsevier: Oxford, UK, 2020; pp. 21–34. ISBN 9780128196618. [Google Scholar]
- Kasim, A.N.; Selamat, M.Z.; Daud, M.A.M.; Yaakob, M.Y.; Putra, A.; Sivakumar, D. Mechanical properties of polypropylene composites reinforced with alkaline treated pineapple leaf fibre from josapine cultivar. Int. J. Automot. Mech. Eng. 2016, 13, 3157–3167. [Google Scholar] [CrossRef]
- Mendes, C.A.D.C.; Adnet, F.A.D.O.; Leite, M.C.A.M.; Furtado, C.R.G.; Sousa, A.M.F. De Chemical, physical, mechanical, thermal and morphological characterization of corn husk residue. Cellul. Chem. Technol. 2014, 49, 727–735. [Google Scholar]
- Yang, J.; Ching, Y.; Chuah, C. Applications of lignocellulosic fibers and lignin in bioplastics: A review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [Green Version]
- Sabaruddin, F.A.; Paridah, M.T.; Sapuan, S.M.; Ilyas, R.A.; Lee, S.H.; Abdan, K.; Mazlan, N.; Roseley, A.S.M.; Abdul Khalil, H.P.S. The effects of unbleached and bleached nanocellulose on the thermal and flammability of polypropylene-reinforced kenaf core hybrid polymer bionanocomposites. Polymers 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Chairani, M.K.; Rizki, M.D.; Mahardika, M.; Handayani, D.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Characterization of compressed bacterial cellulose nanopaper film after exposure to dry and humid conditions. J. Mater. Res. Technol. 2021, 1–25. [Google Scholar] [CrossRef]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Isma, T.; Liu, Q.; Ilyas, R.A.; Lee, C.H. Characterization study of empty fruit bunch (EFB) fibers reinforcement in Poly(Butylene) Succinate (PBS)/Starch/Glycerol Composite Sheet. Polymers 2020, 12, 1571. [Google Scholar] [CrossRef] [PubMed]
- Galindez, A.; Daza, L.D.; Homez-Jara, A.; Eim, V.S.; Váquiro, H.A. Characterization of ulluco starch and its potential for use in edible films prepared at low drying temperature. Carbohydr. Polym. 2019, 215, 143–150. [Google Scholar] [CrossRef]
- Nasser, R.; Salem, M.; Hiziroglu, S.; Al-Mefarrej, H.; Mohareb, A.; Alam, M.; Aref, I. Chemical analysis of different parts of date palm (Phoenix dactylifera L.) using ultimate, proximate and thermo-gravimetric techniques for energy production. Energies 2016, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Ashri, A.; Yusof, M.S.M.; Jamil, M.S.; Abdullah, A.; Yusoff, S.F.M.; Nasir, M.M. Physicochemical characterization of starch extracted from Malaysian wild yam (Dioscorea hispida Dennst.). Emir. J. Food Agric. 2014, 26, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Shujun, W.; Hongyan, L.; Wenyuan, G.; Haixia, C.; Jiugao, Y.; Peigen, X. Characterization of new starches separated from different Chinese yam (Dioscorea opposita Thunb.) cultivars. Food Chem. 2006, 99, 30–37. [Google Scholar] [CrossRef]
- Jiang, Q.; Gao, W.; Li, X.; Xia, Y.; Wang, H.; Wu, S.; Huang, L.; Liu, C.; Xiao, P. Food hydrocolloids characterizations of starches isolated from five different Dioscorea L. species. Food Hydrocoll. 2012, 29, 35–41. [Google Scholar] [CrossRef]
- Bhat, F.M.; Riar, C.S. Effect of amylose, particle size & morphology on the functionality of starches of traditional rice cultivars. Int. J. Biol. Macromol. 2016, 92, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues - Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Fu, N.; Yu, S.J.; Chen, X.D.; Kennedy, J.F. Effects of pulsed electric field treatments on some properties of tapioca starch. Carbohydr. Polym. 2012, 89, 1012–1017. [Google Scholar] [CrossRef] [PubMed]






| Parts of Dioscorea hispida Tubers | References |
|---|---|
| Dioscorea hispida tuber starch-polyacrylamide wood coating characterization | [52] |
| Dioscorea hispida tuber flour | [62] |
| A review on Dioscorea hispida tubers plant | [56] |
| Study on The Starch Granules Morphology | [63] |
| Dioscorea hispida as filler | [64] |
| Modified Dioscorea hispida starch | [58] |
| Tubers as a functional food | [65] |
| Distribution of Dioscorea hispida | [66] |
| Dioscorea hispida starch for edible coating | [67] |
| Chemical Composition of Dioscorea hispida | [53] |
| Natural Starch | Ash (%) | Crude Fat (%) | Crude Protein (%) | Moisture (%) | Starch (%) | Density (g/cm3) |
|---|---|---|---|---|---|---|
| Dioscorea hispida | 2.33 | 0.02 | 5.55 | 9.45 | 37.62 | 1.74 |
| Cassava [70] | 0.31 | - | 0.56 | 12.66 | 58.82 | 1.48 |
| Corn [61] | 0.62 | 7.13 | 7.70 | 10.45 | 79.78 | 1.32 |
| Sugar palm [30] | 0.2 | 0.27 | 0.1 | 9.03 | - | 1.54 |
| Arrowroot [71] | 0.31 | - | ≈0 | 13.20 | 99.32 | - |
| Natural Fibres | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) | Moisture (%) | Density (g/cm3) |
|---|---|---|---|---|---|---|
| Dioscorea hispida | 5.63 | 4.36 | 2.79 | 1.28 | 9.15 | 1.47 |
| Cassava [70] | 10.04 | 29.26 | 3.12 | 3.36 | 14.92 | 1.45 |
| Corn hull [61] | 15.30 | 40.4 | 2.87 | 0.88 | 8.59 | 1.32 |
| Sugar Palm Fibre [7] | 43.88 | 7.24 | 33.24 | 1.01 | 8.36 | 1.50 |
| Oil Palm Fibre [74] | 43.70 | 29.02 | 13.33 | 3.31 | - | - |
| Kenaf [75] | 53.8 | 51.83 | 14.38 | 4 | - | - |
| Sugarcane [76] | 46.0 | 27.0 | 23.0 | - | 8.36 | - |
| Samples | Water Evaporation | 1st Thermal Degradation | 2nd Thermal Degradation | Char Yield | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tonset (°C) | Tmax (°C) | WL (%) | Tonset (°C) | Tmax (°C) | WL (%) | Tonset (°C) | Tmax (°C) | WL (%) | W(%) | |
| DHS | 41.3 | 118.1 | 14.9 | 260.4 | 309.7 | 66.1 | - | - | - | 20.6 |
| DHF | 40.7 | 117.5 | 13.4 | 203.9 | 240.5 | 4.3 | 255.1 | 315.4 | 64.3 | 20.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazrati, K.Z.; Sapuan, S.M.; Zuhri, M.Y.M.; Jumaidin, R. Extraction and Characterization of Potential Biodegradable Materials Based on Dioscorea hispida Tubers. Polymers 2021, 13, 584. https://doi.org/10.3390/polym13040584
Hazrati KZ, Sapuan SM, Zuhri MYM, Jumaidin R. Extraction and Characterization of Potential Biodegradable Materials Based on Dioscorea hispida Tubers. Polymers. 2021; 13(4):584. https://doi.org/10.3390/polym13040584
Chicago/Turabian StyleHazrati, K. Z., S. M. Sapuan, M. Y. M. Zuhri, and R. Jumaidin. 2021. "Extraction and Characterization of Potential Biodegradable Materials Based on Dioscorea hispida Tubers" Polymers 13, no. 4: 584. https://doi.org/10.3390/polym13040584