Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water
Abstract
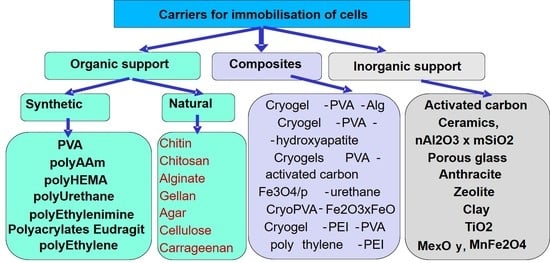
:1. Introduction
2. Application of Immobilised Bacteria
3. Design of Functional Macroporous Cryogels
4. Microorganisms Immobilisation Strategies into Cryogel Structure
5. Applications of Macroporous Cryogels for the Remediation of Contaminants in Water
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Z.; Dong, C.; Liu, Y.; Le, X.; Jin, Z.; Ma, J. Hydro dechlorination and further hydrogenation of 4-chlorophenol to cyclohexanone in water over Pd nanoparticles modified N-doped mesoporous carbon microspheres. Chem. Eng. J. 2015, 270, 215–222. [Google Scholar] [CrossRef]
- Descorme, C. Catalytic wastewater treatment: Oxidation and reduction processes. Recent studies on chlorophenols. Catal. Today 2017, 15, 324–334. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A short review of techniques for phenol removal from wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, J.C.; Doumenq, P.; Guyoneaud, R.; Marrot, B.; Martin-Laurent, F.; Matheron, R.; Moulin, P.; Soulas, G. Applied microbial ecology and bioremediation. In Environmental Microbiology: Fundamentals and Applications; Springer: Dordrecht, The Netherlands, 2015; pp. 659–753. [Google Scholar]
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef] [PubMed]
- Dedov, A.G.; Ivanova, E.A.; Sandzhieva, D.A.; Lobakova, E.S.; Kashcheeva, P.B.; Kirpichnikov, M.P.; Buznik, V.M. New materials and ecology: Biocomposites for aquatic remediation. Theor. Found. Chem. Eng. 2017, 51, 617–630. [Google Scholar] [CrossRef]
- Willaert, R.; Baron, G. Gel entrapment and microencapsulation: Methods, applications and engineering principles. Rev. Chem. Eng. 1996, 12, 1–205. [Google Scholar] [CrossRef]
- Martins, S.C.S.; Martins, C.M.; Fiuza, L.M.C.G.; Santaella, S.T. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial waste water. Afr. J. Biotechnol. 2013, 12, 4412–4418. [Google Scholar]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic compounds in water: Sources, reactivity, toxicity and treatment methods. Phenolic Compd. Nat. Sources Importance Appl. 2017, 420–443. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. C 2020, 114, 111023. [Google Scholar] [CrossRef]
- Lim, J.W.; Zaid, H.F.M.; Isa, M.H.; Oh, W.D.; Adnan, R.; Bashir, M.J.; Wang, D.K. Shielding immobilized biomass cryogel beads with powdered activated carbon for the simultaneous adsorption and biodegradation of 4-chlorophenol. J. Clean. Prod. 2018, 205, 828–835. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Lee, K.; Nahm, C.H.; Jo, S.J.; Lee, J.; Park, P.K. Enhancing the Physical Properties and Lifespan of Bacterial Quorum Quenching Media through Combination of Ionic Cross-Linking and Dehydration. J. Microbiol. Biotechnol. 2017, 27, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Memon, H.; Lanjewar, K.; Dafale, N.; Kapley, A. Immobilization of Microbial Consortia on Natural Matrix for Bioremediation of Wastewaters. Int. J. Environ. Res. 2020, 14, 403–413. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mangwani, N.; Rao, T.S. Bioremediation Approaches for Persistent Organic Pollutants Using Microbial Biofilms. In Microbial Biofilms in Bioremediation and Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2019; pp. 179–206. [Google Scholar]
- Hailei, W.; Ping, L.; Yu, Q.; Hui, Y. Removal of phenol in phenolic resin wastewater by a novel biomaterial: The Phanerochaete chrysosporium pellet containing chlamydospore-like cells. Appl. Microbiol. Biotechnol. 2016, 100, 5153–5164. [Google Scholar] [CrossRef] [PubMed]
- Partovinia, A.; Behnam, R. Review of the immobilized microbial cell systems for bioremediation of petroleum hydrocarbons polluted environments. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1–38. [Google Scholar] [CrossRef]
- Cortez, S.; Nicolau, A.; Flickinger, M.C.; Mota, M. Biocoatings: A new challenge for environmental biotechnology. Biochem. Eng. J. 2017, 121, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Martynenko, N.N.; Gracheva, I.M.; Sarishvili, N.G.; Zubov, A.L.; El’Registan, G.I.; Lozinsky, V.I. Immobilization of champagne yeasts by inclusion into cryogels of polyvinyl alcohol: Means of preventing cell release from the carrier matrix. Appl. Biochem. Microbiol. 2004, 40, 158–164. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Tatarinova, N.Y. The effect of long-term preservation of bacterial cells immobilized in poly(vinyl alcohol) cryogel on their viability and biosynthesis of target metabolites. Microbiology 2007, 76, 336–341. [Google Scholar] [CrossRef]
- Yordanova, G.; Ivanova, D.; Godjevargova, T.; Krastanov, A. Biodegradation of phenol by immobilized Aspergillus awamori NRRL 3112 on modified polyacrylonitrile membrane. Biodegradation 2009, 20, 717–726. [Google Scholar] [CrossRef]
- Von der Ehe, C.; Buś, T.; Weber, C.; Stumpf, S.; Bellstedt, P.; Hartlieb, M.; Schubert, U.S.; Gottschaldt, M. Glycopolymer-functionalized cryogels as catch and release devices for the pre-enrichment of pathogens. Acs Macro Lett. 2016, 5, 326–331. [Google Scholar] [CrossRef]
- Al-Jwaid, A.K.; Berillo, D.; Savina, I.N.; Cundy, A.B.; Caplin, J.L. One-step formation of three-dimensional macroporous bacterial sponges as a novel approach for the preparation of bioreactors for bioremediation and green treatment of water. RSC Adv. 2018, 8, 30813–30824. [Google Scholar] [CrossRef] [Green Version]
- Philip, J.C.; Balmand, S.; Hajto, E.; Bailey, M.J.; Wiles, S.; Whiteley, A.S.; Lilley, A.K.; Hajto, J.; Dunbar, S.A. Whole cell immobilised biosensors for toxicity assessment of a wastewater treatment plant treating phenolics-containing waste. Anal. Chim. Acta 2003, 487, 61–74. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Guan, T.; Guan, J.; Zheng, S.; Chen, B.; Yun, J. Formation dynamics of cell-loading alginate droplets in the micro-tube dripping and cryo-crosslinking process for cell-entrapped cryogel beads as the biocatalysts towards phenyl lactic acid biosynthesis. Ind. Eng. Chem. Res. 2018, 57, 7291–7300. [Google Scholar] [CrossRef]
- Piccirillo, C.; Pereira, S.I.A.; Marques, A.P.; Pullar, R.C.; Tobaldi, D.M.; Pintado, M.E.; Castro, P.M. Bacteria immobilisation on hydroxyapatite surface for heavy metals removal. J. Environ. Manag. 2013, 121, 87–95. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Macroporous gels prepared at subzero temperatures as novel materials for chromatography of particulate-containing fluids and cell culture applications. J. Sep. Sci. 2007, 30, 1657–1671. [Google Scholar] [CrossRef]
- Zaushitsyna, O.; Berillo, D.; Kirsebom, H.; Mattiasson, B. Cryostructured and crosslinked viable cells forming monoliths suitable for bioreactor applications. Top. Catal. 2014, 57, 339–348. [Google Scholar] [CrossRef]
- Berillo, D.A.; Caplin, J.L.; Cundy, A.B.; Savina, I.N. A cryogel-based bioreactor for water treatment applications. Water Res. 2019, 153, 324–334. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Lozinsky, V.; Kolosova, O.; Michurov, D.; Dubovik, A.; Vasil’ev, V.; Grinberg, V. Cryostructuring of Polymeric Systems. 49. Unexpected “Kosmotropic-Like” Impact of Organic Chaotropes on Freeze–Thaw-Induced Gelation of PVA in DMSO. Gels 2018, 4, 81. [Google Scholar] [CrossRef] [Green Version]
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Colloid Interface Sci. 2020, 276, 102088. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzym. Microb. Technol. 1998, 23, 227–242. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Kauanova, S.; Mikhalovsky, S.; Mikhalovska, L.; Saparov, A. Composite Cryogel with Polyelectrolyte Complexes for Growth Factor Delivery. Pharmaceutics 2019, 11, 650. [Google Scholar] [CrossRef] [Green Version]
- Galaev, I.; Dainiak, M.B.; Plieva, F.; Mattiasson, B. Effect of matrix elasticity on affinity binding and release of bioparticles. Elution of bound cells by temperature induced shrinkage of the smart macroporous hydrogel. Langmuir 2007, 23, 35–40. [Google Scholar] [CrossRef]
- Saylan, Y.; Tamahkar, E.; Denizli, A. Recognition of lysozyme using surface imprinted bacterial cellulose nanofibers. J. Biomater. Sci. Polym. Ed. 2017, 28, 1950–1965. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, M.B.; Plieva, F.M.; Galaev, I.Y.; Hatti-Kaul, R.; Mattiasson, B. Cell chromatography: Separation of different microbial cells using IMAC supermacroporous monolithic columns. Biotechnol. Prog. 2005, 21, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Mattiasson, B.; Galaev, I.Y.; Kirsebom, H. Formation of macroporous self-assembled hydrogels through cryogelation of Fmoc–Phe–Phe. J. Colloid Interface Sci. 2012, 368, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, S.; Abe, C.; Hainich, S.; Knolle, W.; Decker, U.; Prager, A.; Konieczny, R. Electron-beam derived polymeric cryogels. Soft Matter 2013, 9, 2484–2492. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Poly-cyclodextrincryogels with aligned porous structure for removal of polycyclic aromatic hydrocarbons (PAHs) from water. J. Hazard. Mater. 2017, 335, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Farías, T.; Hajizadeh, S.; Ye, L. Cryogels with high cisplatin adsorption capacity: Towards removal of cytotoxic drugs from wastewater. Sep. Purif. Technol. 2020, 235, 116203. [Google Scholar] [CrossRef]
- Savina, I.N.; Ingavle, G.C.; Cundy, A.B.; Mikhalovsky, S.V. A simple method for the production of large volume 3D macroporous hydrogels for advanced biotechnological, medical and environmental applications. Sci. Rep. 2016, 6, 21154. [Google Scholar] [CrossRef] [Green Version]
- Berillo, D. Gold nanoparticles incorporated into cryogel walls for decomposition of 4-nitrophenol. J. Clean Prod. 2019, 247, 119089. [Google Scholar] [CrossRef]
- Berillo, D.; Volkova, N. Preparation and physicochemical characteristics of cryogel based on gelatin and oxidised dextran. J. Mater. Sci. 2014, 49, 4855–4868. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, N. Humic acid particle embedded super porous gum Arabic cryogel network for versatile use. Polym. Adv. Technol. 2018, 29, 151–159. [Google Scholar] [CrossRef]
- Zhou, L.; Li, G.; An, T.; Fu, J.; Guoying, S. Recent patents on immobilized microorganism technology and its engineering application in wastewater treatment. Recent Patent Eng. 2008, 2, 28–35. [Google Scholar]
- Choi, M.; Cho, K.; Lee, S.; Chung, Y.C.; Park, J.; Bae, H. Effective seeding strategy using flat type poly (vinyl alcohol) cryogel for anammox enrichment. Chemosphere 2018, 205, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Aleskerova, L.E.; Alenina, K.A.; Efremenko, E.N.; Ismailov, A.D. The factor stabilizing the bioluminescence of PVA-immobilized photobacteria. Microbiology 2017, 86, 218–224. [Google Scholar] [CrossRef]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, H.H.; Lai, C.Y.; Lin, C.W.; Chang, S.H.; Yau, J.T. Microbial community in a pilot-scale biotrickling filter with cell-immobilized biochar beads and its performance in treating toluene-contaminated waste gases. Int. Biodeterior. Biodegrad. 2019, 144, 104743. [Google Scholar] [CrossRef]
- du Toit, J.P.; Pott, R.W. Transparent polyvinyl-alcohol cryogel as immobilisation matrix for continuous biohydrogen production by phototrophic bacteria. Biotechnology for Biofuels. Biotechnol. Biofuels 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Aslıyüce, S.; Denizli, A. Design of PHEMA Cryogel as Bioreactor Matrices for Biological Cyanide Degradation from Wastewater. Hacet. J. Biol. Chem. 2017, 45, 639–645. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. “Deceived” Concentrated Immobilized Cells as Biocatalyst for Intensive Bacterial Cellulose Production from Various Sources. Catalysts 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, T.; Zaman, M.N.; Prasad, B.B.; Shukla, A.; Aggarwal, S.; Singh, R. Rapid immobilization of viable Bacillus pseudomycoides in polyvinyl alcohol/glutaraldehyde hydrogel for biological treatment of municipal wastewater. Environ. Sci. Pollut. Res. 2020, 27, 9167–9180. [Google Scholar] [CrossRef] [PubMed]
- Milakin, K.A.; Capáková, Z.; Acharya, U.; Vajďák, J.; Morávková, Z.; Hodan, J.; Humpolíček, P.; Bober, P. Biocompatible and antibacterial gelatin-based polypyrrole cryogels. Polymer 2020, 197, 122491. [Google Scholar] [CrossRef]
- Wasi, S.; Tabrez, S.; Ahmad, M. Use of Pseudomonas sp. for the bioremediation of environmental pollutants: A review. Environ. Monit. Assess. 2013, 185, 8147–8155. [Google Scholar] [CrossRef]
- Dainiak, M.B.; Kumar, A.; Galaev, I.Y.; Mattiasson, B. Methods in cell separations. In Cell Separation; Mattiasson, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–18. [Google Scholar]
- Bansal, V.; Roychoudhury, P.K.; Mattiasson, B.; Kumar, A. Recovery of urokinase from integrated mammalian cell culture cryogel bioreactor and purification of the enzyme using p-aminobenzamidine affinity chromatography. J. Mol. Recognit. 2006, 19, 332–339. [Google Scholar] [CrossRef]
- Jain, E.; Kumar, A. Disposable polymeric cryogel bioreactor matrix for therapeutic protein production. Nat. Protoc. 2013, 8, 821–835. [Google Scholar] [CrossRef]
- Börner, R.A.; Zaushitsyna, O.; Berillo, D.; Scaccia, N.; Mattiasson, B.; Kirebom, H. Immobilization of Clostridium acetobutylicum DSM 792 as macroporous aggregates through cryogelation for butanol production. Process Biochem. 2014, 49, 10–18. [Google Scholar] [CrossRef]
- Zaushitsyna, O.; Dishisha, T.; Hatti-Kaul, R.; Mattiasson, B. Crosslinked, cryostructured Lactobacillus reuteri monoliths for production of 3-hydroxypropionaldehyde, 3-hydroxypropionic acid and 1,3-propanediol from glycerol. J. Biotechnol. 2017, 241, 22–32. [Google Scholar] [CrossRef]
- Sam, S.P.; Adnan, R.; Ng, S.L. Statistical optimization of immobilization of activated sludge in PVA/alginate cryogel beads using response surface methodology for p-nitrophenol biodegradation. J. Water Process Eng. 2021, 39, 101725. [Google Scholar] [CrossRef]
- Melo, J.S.; Tripathi, A.; Kumar, J.; Mishra, A.; Sandaka, B.P.; Bhainsa, K.C. Immobilization: Then and Now. In Immobilization Strategies; Springer: Singapore, 2021; pp. 1–84. [Google Scholar]
- Williams, S.L.; Eccleston, M.E.; Slater, N.K.H. Affinity capture of biotinylated retrovirus on macroporous monolithic adsorbents: Towards a rapid single-step purification process. Biotechnol. Bioeng. 2005, 89, 783–787. [Google Scholar] [CrossRef]
- Dainiak, M.B.; Galaev, I.Y.; Kumar, A.; Plieva, F.M.; Mattiasson, B. Chromatography of living cells using supermacroporous hydrogels, cryogels. Adv. Biochem. Eng. Biotechnol. 2007, 106, 101–127. [Google Scholar]
- Sharma, A.; Bhat, S.; Vishnoi, T.; Nayak, V.; Kumar, A. Three-dimensional supermacroporous carrageenan-gelatin cryogel matrix for tissue engineering applications. BioMed Res. Int. 2013, 2013, 478279. [Google Scholar] [CrossRef] [Green Version]
- Eichhorn, T.; Ivanov, A.E.; Dainiak, M.B.; Leistner, A.; Linsberger, I.; Jungvid, H.; Mikhalovsky, S.V.; Weber, V. Macroporous Composite Cryogels with Embedded polystyrene Divinylbenzene Microparticles for the Adsorption of Toxic Metabolites from Blood. J. Chem. 2013, 2013, 348412. [Google Scholar] [CrossRef] [Green Version]
- Baydemir, G.; Andaç, M.; Perçin, I.; Derazshamshir, A.; Denzil, A. Molecularly imprinted composite cryogels for hemoglobin depletion from human blood. J. Mol. Recognit. 2013, 27, 528–536. [Google Scholar] [CrossRef]
- Erol, K. Polychelatedcryogels: Hemoglobin adsorption from human blood. Artif. Cells Nanomed. Biotechnol. 2017, 45, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plieva, F.M.; Mattiasson, B. Macroporous Gel Particles as Novel Sorbent Materials: Rational Design. Ind. Eng. Chem. Res. 2008, 47, 4131–4141. [Google Scholar] [CrossRef]
- Rusten, B.; Eikebrokk, B.; Ulgenes, Y.; Lygren, E. Design and Operations of the Kaldnes Moving Bed Biofilm Reactors. Aquac. Eng. 2006, 34, 322–331. [Google Scholar] [CrossRef]
- Ødegaard, H.; Rusten, B.; Westrum, T. A new moving bed biofilm reactor—Applications and results. Water Sci. Technol. 1994, 29, 157–165. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Vo, D.V.N.; Tran, N.H. Biocarriers for biofilm immobilization in wastewater treatments: A review. Environ. Chem. Lett. 2020, 18, 1925–1945. [Google Scholar] [CrossRef]
- Önnby, L.; Giorgi, C.; Plieva, F.M.; Mattiasson, B. Removal of heavy metals from water effluents using supermacroporous metal chelating cryogels. Biotechnol. Prog. 2010, 26, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Nkemka, V.N.; Murto, M. Evaluation of biogas production from seaweed in batch tests and in UASB reactors combined with the removal of heavy metals. J. Environ. Manag. 2010, 91, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Kirsebom, H.; Mattiasson, B.; Galaev, I.Y. Building macroporous materials from microgels and microbes via one-step cryogelation. Langmuir 2009, 25, 8462–8465. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; van Langen, L.M.; van Rantwijk, F.; Sheldon, R.A. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol. Bioeng. 2004, 86, 273–276. [Google Scholar] [CrossRef]
- Bečka, S.; Škrob, F.; Plháčková, K.; Kujan, P.; Holler, P.; Kyslík, P. Cross-linked cell aggregates of Trigonopsis variabilis: D-amino acid oxidase catalyst for oxidation of cephalosporin C. Biotechnol. Lett. 2003, 25, 227–233. [Google Scholar] [CrossRef] [PubMed]
- De Alteriis, E.; Parascandola, P.; Seardi, V. Oxidized starch as a hardening agent in the gelatin-immobilization of living yeast cells. Starch/Stärke 1990, 42, 57–60. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Kube, M.; Jefferson, B.; Fan, L.; Roddick, F. The impact of wastewater characteristics, algal species selection and immobilisation on simultaneous nitrogen and phosphorus removal. Algal Res. 2018, 31, 478–488. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Chen, L.; Ren, Q.; Cai, S.; Zhang, J. The biodegradation pathway of triethylamine and its biodegradation by immobilized Arthrobacter protophormiae cells. J. Hazard. Mater. 2011, 186, 59–66. [Google Scholar] [CrossRef]
- Le Noir, M.; Plieva, F.M.; Mattiasson, B. Removal of endocrine-disrupting compounds from water using macroporous molecularly imprinted cryogels in a moving-bed reactor. J. Sep. Sci. 2009, 32, 1471–1479. [Google Scholar] [CrossRef]
- See, S.; Lim, P.-E.; Lim, J.-W.; Seng, C.-E.; Adnan, R. Evaluation of o-cresol removal using PVA-cryogel-immobilised biomass enhanced by PAC. Water SA 2005, 41, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Inglezakis, V.J.; Kudarova, A.; Tarassov, D.; Jetybayeva, A.; Myngtay, Y.; Zhalmuratova, D.; Nurmukhambetov, D. Inhibitory effects of polar and non-polar organic substances on activated sludge activity. Desalin. Water Treat. 2017, 91, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Stylianou, M.A.; Kollia, D.; Haralambous, K.J.; Inglezakis, V.J.; Moustakas, K.G.; Loizidou, M.D. Effect of acid treatment on the removal of heavy metals from sewage sludge. Desalination 2007, 215, 73–81. [Google Scholar] [CrossRef]
- Alessandrello, M.J.; Tomás, M.S.J.; Isaac, P.; Vullo, D.L.; Ferrero, A. Use of Immobilized Biomass as Low-Cost Technology for Bioremediation of PAHs Contaminated Sites. In Strategies for Bioremediation of Organic and Inorganic Pollutants; CRC Press: Boca Raton, FL, USA, 2018; pp. 67–81. [Google Scholar]
- Serebrennikova, M.K.; Golovina, E.E.; Kuyukina, M.S.; Ivshina, I.B. A consortium of immobilized Rhodococci for oil field wastewater treatment in a column bioreactor. Appl. Biochem. Microbiol. 2017, 53, 435–440. [Google Scholar] [CrossRef]
- Sahin, Z.M.; Alimli, D.; Tonta, M.M.; Kose, M.E.; Yilmaz, F. Highly sensitive and reusable mercury (II) sensor based on fluorescence quenching of pyrene moiety in polyacrylamide-based cryogel. Sens. Actuators B Chem. 2017, 242, 362–368. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Lu, H.; Li, G.; Huang, Q. Lamp: A database linking antimicrobial peptides. PLoS ONE 2013, 8, e66557. [Google Scholar] [CrossRef] [Green Version]
- Brackett, D.J.; Lerner, M.R.; Lacquement, M.A.; He, R.; Pereira, H.A. A synthetic lipopolysaccharide-binding peptide based on the neutrophil-derived protein cap37 prevents endotoxin-induced responses in conscious rats. Infect. Immun. 1997, 65, 2803–2811. [Google Scholar] [CrossRef] [Green Version]
- Shirbin, S.J.; Shu, J.; Lam, S.J.; Chan, N.J.-A.; Ozmen, M.M.; Fu, Q.; O’Brien-Simpson, N.; Reynolds, E.C.; Qiao, G.G. Polypeptide-Based Macroporous Cryogels with Inherent Antimicrobial Properties: The Importance of a Macroporous Structure. ACS Macro Lett. 2016, 5, 552–557. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Union 2006, 396, 1–849. [Google Scholar]
- Pembrey, R.S.; Marshall, K.C.; Schneider, R.P. Cell surface analysis techniques: What do cell preparation protocols do to cell surface properties? Appl. Environ. Microbiol. 1999, 65, 2877–2894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leenen, E.J.; Dos Santos, V.A.; Grolle, K.C.; Tramper, J.; Wijffels, R. Characteristics of and selection criteria for support materials for cell immobilization in wastewater treatment. Water Res. 1996, 30, 2985–2996. [Google Scholar] [CrossRef]
- Choi, M.; Chaudhary, R.; Lee, M.; Kim, J.; Cho, K.; Chung, Y.C.; Bae, H.; Park, J. Enhanced selective enrichment of partial nitritation and anammox bacteria in a novel two-stage continuous flow system using flat-type poly(vinylalcohol) cryogel films. Bioresour. Technol. 2020, 300, 122546. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Liu, X.; Su, Y. Effects of biomass and environmental factors on nitrogen removal performance and community structure of an anammox immobilized filler. Sci. Total Environ. 2020, 710, 135258. [Google Scholar] [CrossRef]
- Dolejš, I.; Stloukal, R.; Rosenberg, M.; Rebroš, M. Nitrogen removal by co-immobilized anammox and ammonia-oxidizing bacteria in wastewater treatment. Catalysts 2019, 9, 523. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Zeng, Z.T.; Niu, Q.Y.; Xiao, R.; Zeng, G.M.; Liu, Y.; Cheng, M.; Hu, K.; Jiang, L.H.; Tan, X.F.; et al. Influence of immobilization on phenanthrene degradation by Bacillus sp. P1 in the presence of Cd (II). Sci. Total Environ. 2019, 655, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Radosavljević, M.; Lević, S.; Belović, M.; Pejin, J.; Djukić-Vuković, A.; Mojović, L.; Nedović, V. Immobilization of Lactobacillus rhamnosus in polyvinyl alcohol/calcium alginate matrix for production of lactic acid. Bioprocess Biosyst. Eng. 2020, 43, 315–322. [Google Scholar] [CrossRef]
- Christova, N.; Kabaivanova, L.; Nacheva, L.; Petrov, P.; Stoineva, I. Biodegradation of crude oil hydrocarbons by a newly isolated biosurfactant producing strain. Biotechnol. Biotechnol. Equip. 2019, 33, 863–872. [Google Scholar] [CrossRef]
- Tekere, M. Microbial Bioremediation and Different Bioreactors Designs Applied. In Biotechnology and Bioengineering; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Husain, Q. Remediation of phenolic compounds from polluted water by immobilized peroxidases. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Singapore, 2019; pp. 329–358. [Google Scholar]
- Dzionek, A.; Wojcieszyńska, D.; Adamczyk-Habrajska, M.; Karczewski, J.; Potocka, I.; Guzik, U. Xanthan gum as a carrier for bacterial cell entrapment: Developing a novel immobilised biocatalyst. Mater. Sci. Eng. C 2021, 118, 111474. [Google Scholar] [CrossRef]
- Prabu, C.S.; Thatheyus, A. Biodegradation of acrylamide employing free and immobilized cells of Pseudomonas aeruginosa. Int. Biodeterior. Biodegrad. 2007, 60, 269–273. [Google Scholar]
- Satchanska, G.; Topalova, Y.; Dimkov, R.; Groudeva, V.; Petrov, P.; Tsvetanov, C.; Selenska-Pobell, S.; Golovinsky, E. Phenol degradation by environmental bacteria entrapped in cryogels. Biotechnol. Biotechnol. Equip. 2015, 29, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Mrudula, S.; Shyam, N. Immobilization of Bacillus megaterium MTCC 2444 by Ca-alginate entrapment method for enhanced alkaline protease production. Braz. Arch. Biol. Technol. 2012, 55, 135–144. [Google Scholar] [CrossRef]
- Otero-González, L.; Mikhalovsky, S.V.; Václavíková, M.; Trenikhin, M.V.; Cundy, A.B.; Savina, I.N. Novel nanostructured iron oxide cryogels for arsenic (As (III)) removal. J. Hazard. Mater. 2020, 381, 120996. [Google Scholar] [CrossRef] [PubMed]
- Önnby, L.; Pakade, V.; Mattiasson, B.; Kirsebom, H. Polymer composite adsorbents using particles of molecularly imprinted polymers or aluminium oxide nanoparticles for treatment of arsenic contaminated waters. Water Res. 2012, 46, 4111–4120. [Google Scholar] [CrossRef]
- Baimenov, A.Z.; Berillo, D.A.; Moustakas, K.; Inglezakis, V.J. Efficient removal of mercury (II) from water by use of cryogels and comparison to commercial adsorbents under environmentally relevant conditions. J. Hazard. Mater. 2020, 399, 123056. [Google Scholar] [CrossRef]
- Baimenov, A.; Berillo, D.; Azat, S.; Nurgozhin, T.; Inglezakis, V. Removal of Cd2+ from water by use of super-macroporous cryogels and comparison to commercial adsorbents. Polymers 2020, 12, 2405. [Google Scholar] [CrossRef] [PubMed]
- Baimenov, A.Z.; Berillo, D.A.; Inglezakis, V.J. Cryogel-based Ag/Ag2O nanocomposites for iodide removal from water. J. Mol. Liq. 2020, 299, 112134. [Google Scholar] [CrossRef]
- Hajizadeh, S.; Kirsebom, H.; Galaev, I.Y.; Mattiasson, B. Evaluation of selective composite cryogel for bromate removal from drinking water. J. Sep. Sci. 2010, 33, 1752–1759. [Google Scholar] [CrossRef]
- Idris, Z.M.; Hameed, B.H.; Ye, L.; Hajizadeh, S.; Mattiasson, B.; Din, A.M. Amino-functionalised silica-grafted molecularly imprinted polymers for chloramphenicol adsorption. J. Environ. Chem. Eng. 2020, 8, 103981. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Immobilization of cesium-resistant bacterial cells by radiation polymerization and their bioremoval efficiency. Water Sci. Technol. 2019, 79, 1587–1596. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Zhang, T.; Zhou, X.; Li, C. Preparation of magnetic macroporous polymer sphere for biofilm immobilization and biodesulfurization. React. Funct. Polym. 2019, 141, 1–8. [Google Scholar] [CrossRef]
- Kilonzo, P.; Bergougnou, M. Surface modifications for controlled and optimized cell immobilization by adsorption: Applications in fibrous bed bioreactors containing recombinant cells. J. Microbiol. Biochem. Technol. 2012, 4, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Busalmen, J.P.; Sanchez, S.R. Influence of pH and ionic strength on adhesion of a wild strain of Pseudomonas sp. to titanium. J. Ind. Microbiol. Biotechnol. 2001, 26, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.; Hirasawa, J.S.; Varesche, M.B.; Foresti, E.; Zaiat, M. Evaluation of support materials for the immobilization of sulphate-reducing bacteria and methanogenic archaea. Anaerobe 2006, 12, 93–98. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Diao, X.; Luo, G. Effect of pore diameter and cross-linking method on the immobilization efficiency of Candida rugose lipase in SBA-15. Bioresour. Technol. 2010, 101, 3830–3837. [Google Scholar] [CrossRef] [PubMed]





| Microorganism | Polymer Type or Cross-Linker | Application | Reference |
|---|---|---|---|
| Aspergillus awamori | polyacrylonitrile membrane | phenol biodegradation | [21] |
| Clostridium acetobutylicum, E. coli, Pseudomonas spp., Rhodococcus spp. | glutaraldehyde | biofuel, bioremediation | [23,62,78] |
| E. coli, Clostridium acetobutylicum, Pseudomonas spp., Rhodococcus spp., Acinetobacter spp. | PVA-aldehyde/PEI aldehyde/oxidized dextran or aldehyde dextran | hydrolysis/fermentation/bioremediation | [23,28,29,62] |
| Actinobacillus succinogenes, Rhizopusoryzae spp. | PVA-cryogel | lactic, fumaric and succinic acids | [51] |
| Chlorella spp. | Alg, Carrageenan, Agarose, Alginate and Agar beads, polyurethane foam | removal of ions Ni, Zn, Cd, Cu, Hg, Pb, and uranium, phosphate, nitrite, NH4 | [52,111,112,113] |
| Pseudomonas citronellolis | PVA bamboo-biochar beads | toluene and hydrocarbons | [46] |
| Trichoderma spp. | HEMA cryogel | cyanide removal | [55] |
| Komagataei bacterxylinum | PVA-cryogel | microcellulose | [58] |
| - | polylysine-b-polyvaline GA cryogel Gum Arabic linked with divinyl sulfone | water treatment, antimicrobial activity E-coli | [54] |
| - | aldehyde modified dextran | scaffolds or mammalian cell immobilisation | [28,82] |
| Pseudomonas fluorescens(S3X), Microbacterium oxydans (EC29) | hydroxyapatite | removal of zinc and cadmium ions | [68] |
| Pseudomonas rhodesiae, Bacillus subtilis, Bacillus lateroporus | cryogel polyethylenoxide UV | phenol, methylphenol/cresol remediation | [85,86,122] |
| Nitrosomonas europaea C-31 and ‘Candidatus Jettenia caeni, Rhodococcus spp., Bacillus sp., Pseudomonas spp. | PVA cryogel | removal of ammonia | [90,98] |
| Bacillus | PVA- H3BO3-Ca- Alg beads | polycyclic aromatic hydrocarbons removal | [101] |
| Lactobacillus rhamnosus | PVA-Alg-Ca cryogel | lactate production | [103] |
| Bacillus cereus | pAAm-BisAAm cryogel | crude oil degradation | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. https://doi.org/10.3390/polym13071073
Berillo D, Al-Jwaid A, Caplin J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers. 2021; 13(7):1073. https://doi.org/10.3390/polym13071073
Chicago/Turabian StyleBerillo, Dmitriy, Areej Al-Jwaid, and Jonathan Caplin. 2021. "Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water" Polymers 13, no. 7: 1073. https://doi.org/10.3390/polym13071073