Preparation, Properties, and Microbial Impact of Tungsten (VI) Oxide and Zinc (II) Oxide Nanoparticles Enriched Polyethylene Sebacate Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Nano-Oxides Fabrication
2.3. Nanocomposite Fabrication
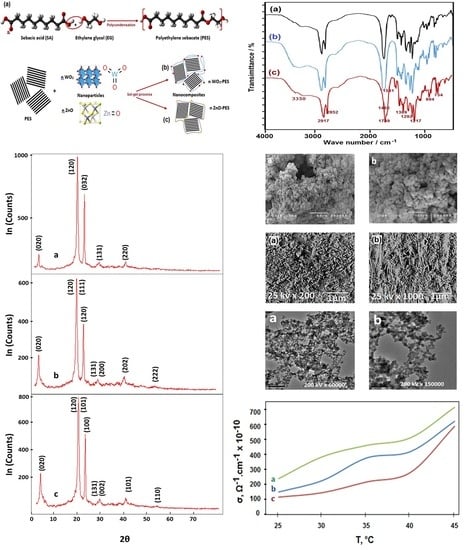
2.4. Characterization Techniques
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, C.; Bhardwaj, A. Summary and future perspectives of nanomaterials and technologies: Special emphasis on energy and environment. In Nanomaterials for Sustainable Energy and Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–353. [Google Scholar]
- Pathakoti, K.; Manubolu, M.; Hwang, H.-M. Nanotechnology applications for environmental industry. In Handbook of Nano Materials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; p. 894. [Google Scholar]
- Dong, P.; Prasanth, R.; Xu, F.; Wang, X.; Li, B.; Shankar, R. Eco-friendly polymer nanocomposite-properties and processing. In Eco-Friendly Polymer Nanocomposites; Thakur, V., Thakur, M., Eds.; Springer: New Delhi, India, 2015; Volume 75, p. 1. [Google Scholar]
- Boyaciyan, D.; von Klitzing, R. Stimuli-responsive polymer/metal composites: From fundamental research to self-regulating devices. Curr. Opin. Colloid Interface Sci. 2019, 44, 193–207. [Google Scholar] [CrossRef]
- Jimmy, J.; Kandasubramanian, B. MXene functionalized polymer composites: Synthesis and applications. Eur. Polym. J. 2020, 122, 109367. [Google Scholar] [CrossRef]
- Biswal, T.; BadJena, S.K.; Pradhan, D. Synthesis of polymer composite materials and their biomedical applications. Mater. Today Proc. 2020, 30, 305–315. [Google Scholar] [CrossRef]
- Rawat, M.K.; Kukreja, N.; Gupta, S.K. Effect of reinforcing micro sized aluminium oxide particles on mechanical properties of polymer based composite. Mater Today Proc. 2020, 26, 1306–1309. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Song, H.; Oh, Y.; Jeong, Y. Synthesis of sulfur-co-polymer/porous long carbon nanotubes composite cathode by chemical and physical binding for high performance lithium-sulfur batteries. Energy 2020, 195, 117034. [Google Scholar] [CrossRef]
- Wooh, S.; Encinas, N.; Vollmer, D.; Butt, H.-J. Stable hydrophobic metal-oxide photocatalysts via grafting polydimethylsiloxane brush. Adv. Mater. 2017, 29, 1604637. [Google Scholar] [CrossRef] [PubMed]
- With, P.C.; Helmstedt, U.; Prager, L. Flexible transparent barrier applications of oxide thin films prepared by photochemical conversion at low temperature and ambient pressure. Front. Mater. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Neves, A.C.C.; Rohen, L.A.; Mantovani, D.P.; Carvalho, J.P.R.G.; Vieira, C.M.F.; Lopes, F.P.D.; Monteiro, S.N. Comparative mechanical properties between biocomposites of epoxy and polyester matrices reinforced by hemp fiber. J. Mater. Res. Technol. 2019, 9, 1296–1304. [Google Scholar] [CrossRef]
- Rajesh, G.; Revuri, A.; Arekapudi, M.S.; DBM, G.R. Evaluating tensile properties of phragmites karka fibre reinforced polyester composites. Mater. Today Proc. 2019, 18, 8–14. [Google Scholar] [CrossRef]
- Ezahra, T.F.; Abderrahmane, E.I.; Reda, B.; Abdeslam, A.; Mohamed, A.; Soufian, E.B.; Abdesselam, T. Cellulose grafted aliphatic polyesters: Synthesis, characterization and biodegradation under controlled conditions in a laboratory test system. J. Mol. Struct. 2019, 1205, 127582. [Google Scholar]
- Li, S.; Liu, Z.; Hou, L.; Chen, Y.; Xu, T. Effect of polyether/polyester polyol ratio on properties of waterborne two-component polyurethane coatings. Prog. Org. Coat. 2020, 141, 105545. [Google Scholar] [CrossRef]
- Ismail, A.S. Interactive effect of ethylene diamine in preparation of chitosan-based poly(ester-urethane) elastomers. Int. J. Sci. Eng. Technol. 2015, 3, 1340–1343. [Google Scholar]
- Verma, D.K.; Purohit, R.; Rana, R.S.; Purohit, S.; Patel, K.K. Enhancement of the properties of shape memory polymers using different nano size reinforcement—A review. Mater. Today Proc. 2020, 26, 3037–3042. [Google Scholar] [CrossRef]
- Kartik Shubham, S.; Purohit, R.; Yadav, P.S.; Rana, R.S. Study of nano-fillers embedded in polymer matrix composites to enhance its properties—A review. Mater. Today Proc. 2020, 26, 3024–3029. [Google Scholar] [CrossRef]
- Guo, W.; Xu, L.; Feng, P.; Gu, Y.; Shuai, C. In-situ growth of silica nano-protrusions on halloysite nanotubes for interfacial reinforcement in polymer/halloysite scaffolds. Appl. Surf. Sci. 2020, 513, 145772. [Google Scholar] [CrossRef]
- Han, T.; Nagarajan, S.; Zhao, H.; Sun, C.; Wen, S.; Zhao, S.; Zhang, L. Novel reinforcement behavior in nanofilled natural rubber (NR) / butadiene-acrylonitrile rubber (NBR) blends: Filling-polymer network and supernanosphere. Polymer 2019, 186, 122005. [Google Scholar] [CrossRef]
- Jeong, J.W.; Hwang, H.S.; Choi, D.; Ma, B.C.; Jung, J.; Chang, M. Hybrid polymer/metal oxide thin films for high performance, flexible transistors. Micromachines 2020, 11, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.M.; Dat, D.Q.; Van Duy, N.; Van Quang, V.; Van Toan, N.; Van Hieu, N.; Hoa, N.D. Facile synthesis of ultrafine rGO/WO3 nanowire nanocomposites for highly sensitive toxic NH3 gas sensors. Mater. Res. Bull. 2020, 125, 110810. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Qiu, L.; Rasaki, S.A.; Qu, F.; Thomas, T.; Yang, M. Ru-decorated WO3 nanosheets for efficient xylene gas sensing application. J. Alloys Compd. 2020, 826, 154196. [Google Scholar] [CrossRef]
- Younas, M.; Gondal, M.A.; Dastageer, M.A.; Baig, U. Fabrication of cost effective and efficient dye sensitized solar cells with WO3-TiO2 nanocomposites as photoanode and MWCNT as Pt-free counter electrode. Ceram. Int. 2018, 45, 936–947. [Google Scholar] [CrossRef]
- Kim, H.; Ryu, J.H.; Kim, J.; Hwang, K.; Kang, H.; Oh, S.M.; Yoon, S. Ordered mesoporous tungsten oxide-carbon nanocomposite for use as a highly reversible negative electrode in lithium-ion batteries. J. Alloys Compd. 2020, 832, 154816. [Google Scholar] [CrossRef]
- Oderinde, O.; Hussain, I.; Kang, M.; Wu, Y.; Mulenga, K.; Adebayo, I.; Fu, G. Water as DES-cosolvent on the morphology tuning and photochromic enhancement of tungsten oxide-molybdenum oxide nanocomposite. J. Ind. Eng. Chem. 2019, 80, 1–10. [Google Scholar] [CrossRef]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N. Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J. Phys. Chem. Solids 2018, 116, 137–147. [Google Scholar] [CrossRef]
- Renukadevi, R.; Sundaram, R. Synthesis, characterization, humidity sensing, antibacterial, photocatalytic and kinetic studies of novel HgWO4-WO3 nanocomposites. Mater. Today Proc. 2019, 8, 153–161. [Google Scholar] [CrossRef]
- Pawar, S.M.; Pawar, B.S.; Babar, P.T.; Ahmed, A.T.A.; Chavan, H.S.; Jo, Y.; Cho, S.; Kim, J.; Hou, B.; Inamdar, A.I.; et al. Nanoporous CuCo2O4 nanosheets as a highly efficient bifunctional electrode for supercapacitors and water oxidation catalysis. Appl. Surf. Sci. 2019, 470, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.G.; Yardani Sefidi, P.; Musap Mert, A.; Kinayyigit, S. Investigation of solar-induced photoelectrochemical water splitting and photocatalytic dye removal activities of camphor sulfonic acid doped polyaniline-WO3-MWCNT ternary nanocomposite. J. Mater. Sci. Technol. 2019, 38, 7–18. [Google Scholar] [CrossRef]
- Sekar, S.; Talha Aqueel Ahmed, A.; Pawar, S.M.; Lee, Y.; Im, H.; Young Kim, D.; Lee, S. Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl. Surf. Sci. 2019, 508, 145127. [Google Scholar] [CrossRef]
- Beyhaqi, A.; Zeng, Q.; Chang, S.; Wang, M.; Taghi Azimi, S.M.; Hu, C. Construction of g-C3N4/WO3/MoS2 ternary nanocomposite with enhanced charge separation and collection for efficient wastewater treatment under visible light. Chemosphere 2020, 247, 125784. [Google Scholar] [CrossRef]
- Tijani, J.O.; Ugochukwu, O.; Fadipe, L.A.; Bankole, M.T.; Abdulkareem, A.S.; Roos, W.D. Photocatalytic degradation of local dyeing wastewater by iodine-phosphorus co-doped tungsten trioxide nanocomposites under natural sunlight irradiation. J. Environ. Manag. 2019, 236, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Zargoosh, K.; Moradi Aliabadi, H. SrAl2O4:Eu2+: Dy3+/WO3/polyester nanocomposite as a highly efficient and environmentally friendly photocatalyst for removal of dyes from industrial wastes. Environ. Nanotechnol. Monit. 2019, 12, 100273. [Google Scholar] [CrossRef]
- Zhao, Q.; Fang, Y.; Qiao, K.; Wei, W.; Yao, Y.; Gao, Y. Printing of WO3/ITO nanocomposite electrochromic smart windows. Sol. Energy Mater. Sol. Cells 2019, 194, 95–102. [Google Scholar] [CrossRef]
- Devaraju, A.; Sivasamy, P.; Loganathan, G.B. Mechanical properties of polymer composites with ZnO nano-particle. Mater. Today Proc. 2019, 22, 531–534. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y.; Liu, S. Synthesis of Fe2O3–ZnWO4 nanocomposites and their enhanced acetone sensing performance. J. Alloys Compd. 2020, 831, 154713. [Google Scholar] [CrossRef]
- Habib, M.A.; Shahadat, M.T.; Bahadur, N.M.; Ismail, I.M.; Mahmood, A. Synthesis and characterization of ZnO-TiO2 nanocomposites and their application as photocatalysts. Int. Nano Lett. 2013, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ramezani Darabi, R.; Jahanshahi, M.; Peyravi, M. A support assisted by photocatalytic Fe3O4/ZnO nanocomposite for thin-film forward osmosis membrane. Chem. Eng. Res. Des. 2018, 133, 11–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Guo, B. Photodegradation behavior of poly(butylene succinate-co-butylene adipate)/ZnO nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 173–181. [Google Scholar] [CrossRef]
- Amani, A.; Montazer, M.; Mahmoudirad, M. Synthesis of applicable hydrogel corn silk/ZnO nanocomposites on polyester fabric with antimicrobial properties and low cytotoxicity. Int. J. Biol. Macromol. 2018, 123, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Vasuki, K.; Manimekalai, R. NIR light active ternary modified ZnO nanocomposites for combined cancer therapy. Heliyon 2019, 5, e02729. [Google Scholar] [CrossRef]
- Bužarovska, A.; Dinescu, S.; Lazar, A.D.; Serban, M.; Pircalabioru, G.G.; Costache, M.; Avérous, L. Nanocomposite foams based on flexible biobased thermoplastic polyurethane and ZnO nanoparticles as potential wound dressing materials. Mater. Sci. Eng. C 2019, 104, 109893. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; He, C.; Wang, H. Fabrication of antimicrobial films based on hydroxylethylcellulose and ZnO for food packaging application. Food Packag. Shelf Life 2020, 23, 100462. [Google Scholar] [CrossRef]
- Sreedhar, A.; Reddy, I.N.; Hoai Ta, Q.T.; Namgung, G.; Cho, E.; Noh, J.-S. Facile growth of novel morphology correlated Ag/Co-doped ZnO nanowire/flake-like composites for superior photoelectrochemical water splitting activity. Ceram. Int. 2019, 45, 6985–6993. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C. Mxene enhanced the photocatalytic activity of ZnO nanorods under visible light. Mater. Lett. 2020, 261, 127127. [Google Scholar] [CrossRef]
- Chen, C.; Mei, W.; Wang, C.; Yang, Z.; Chen, X.; Chen, X.; Liu, T. Synthesis of a flower-like SnO/ZnO nanostructure with high catalytic activity and stability under natural sunlight. J. Alloys Compd. 2020, 826, 154122. [Google Scholar] [CrossRef]
- Yulizar, Y.; Apriandanu, D.O.B.; Ashna, R.I. La2CuO4-decorated ZnO nanoparticles with improved photocatalytic activity for malachite green degradation. Chem. Phys. Lett. 2020, 755, 137749. [Google Scholar] [CrossRef]
- Dedova, T.; Acik, I.O.; Chen, Z.; Katerski, A.; Balmassov, K.; Gromyko, I.; Nagyne-Kovacs, T.; Szilagyi, I.M.; Krunks, M. Enhanced photocatalytic activity of ZnO nanorods by surface treatment with HAuCl4: Synergic effects through an electron scavenging, plasmon resonance and surface hydroxylation. Mater. Chem. Phys. 2020, 245, 122767. [Google Scholar] [CrossRef]
- Ismail, A.S.; Mady, A.H.; Tawfik, S.M. Synthesis, characterization and biological activity of iron (III) oxide and titanium (IV) oxide nanoparticle dispersed polyester resin nanocomposites. Arab. J. Sci. Eng. 2019, 45, 197–203. [Google Scholar] [CrossRef]
- Corrie, C.L.; Klabunde, K.J. The catalytic methanol synthesis over nanoparticle metal oxide catalysts. J. Mol. Catal. A Chem. 2003, 194, 227–237. [Google Scholar]
- Hoseini, F.; Farahamndjou, M.; Firoozabadi, T.P. Coprecipitation synthesis of zinc ferrit (Fe2O3/ZnO) nanoparticles prepared by CTAB surfactant. J. Fundam. Appl. Sci. 2016, 8, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Wu, D.; Fu, R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Tsanaktsis, V.; Nikolaidis, N.; Kostoglou, M.; Bikiaris, D.N. Effect of catalyst type on molecular weight increase and coloration of poly(ethylene furanoate) biobased polyester during melt polycondensation. Polym. Chem. 2017, 8, 6895–6908. [Google Scholar] [CrossRef]
- Ni, Z.; Li, F.; Wang, H.; Wang, S.; Gao, S. Catalytic esterification, kinetics, and cold flow properties of isobutyl palmitate. Fuel 2019, 254, 115368. [Google Scholar] [CrossRef]
- Zia, K.M.; Zuber, M.; Barikani, M.; Jabbar, A.; Khosa, M.K. XRD pattern of chitin based polyurethane bio-nanocomposites. Carbohydr. Polym. 2010, 80, 539–543. [Google Scholar] [CrossRef]
- Nagarjuna, R.; Challagulla, S.; Sahu, P.; Roy, S.; Ganesan, R. Polymerizable sol–gel synthesis of nano-crystalline WO3 and its photocatalytic Cr(VI) reduction under visible light. Adv. Powder Technol. 2017, 28, 3265–3273. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Gavara, R.; Hernández-Muñoz, P. Diffusion modeling in polymer–clay nanocomposites for food packaging applications through finite element analysis of TEM images. J. Membr. Sci. 2015, 482, 92–102. [Google Scholar] [CrossRef]
- Momohjimoh, I.; Saheb, N.; Hussein, M.A.; Laoui, T.; Al-Aqeeli, N. Electrical conductivity of spark plasma sintered Al2O3–SiC and Al2O3-carbon nanotube nanocomposites. Ceram. Int. 2020, 46, 16008–16019. [Google Scholar] [CrossRef]
- Vitta, Y.; Figueroa, M.; Calderon, M.; Ciangherotti, C. Synthesis of iron nanoparticles from aqueous extract of Eucalyptus robusta Sm and evaluation of antioxidant and antimicrobial activity. Mat. Sci. Energy Technol. 2019, 3, 97–103. [Google Scholar] [CrossRef]
- Salton, M.R.J. Cell Membrane Transport: Principles and Techniques. J. Gen. Physiol. 1968, 52, 277–299. [Google Scholar]
- Zaki, M.F.; Tawfik, S.M. Synthesis, surface properties and antimicrobial activity of some germanium nonionic surfactants. J. Oleo Sci. 2014, 63, 921–931. [Google Scholar] [CrossRef] [Green Version]







| No. | Retention Time | Mn | Mw | MP (Daltons) | Mz (Daltons) | Mz + 1 (Daltons) | Poly- Dispersity |
|---|---|---|---|---|---|---|---|
| 1 | 28.883 | 2103 | 2621 | 2422 | 3240 | 3888 | 1.246 |
| Test Organism | (+ve) Bacteria | (-ve) Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|---|
| Compound ID | Bacillus subtilis | Staph. aureus | Escherichia coli | Pseud. aeruginosa | Candida albicans | Aspergillus niger | |
| PES | 13 | 18 | 12 | 12 | 28 | 25 | |
| PES-WO3 | 13 | 16 | 13 | 13 | 17 | 20 | |
| PES-ZnO | 12 | 17 | 13 | 12 | 19 | 19 | |
| Reference | 32 | 34 | 32 | 33 | 26 | 28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, A.S.; Tawfik, S.M.; Mady, A.H.; Lee, Y.-I. Preparation, Properties, and Microbial Impact of Tungsten (VI) Oxide and Zinc (II) Oxide Nanoparticles Enriched Polyethylene Sebacate Nanocomposites. Polymers 2021, 13, 718. https://doi.org/10.3390/polym13050718
Ismail AS, Tawfik SM, Mady AH, Lee Y-I. Preparation, Properties, and Microbial Impact of Tungsten (VI) Oxide and Zinc (II) Oxide Nanoparticles Enriched Polyethylene Sebacate Nanocomposites. Polymers. 2021; 13(5):718. https://doi.org/10.3390/polym13050718
Chicago/Turabian StyleIsmail, Amr S., Salah M. Tawfik, Amr H. Mady, and Yong-Ill Lee. 2021. "Preparation, Properties, and Microbial Impact of Tungsten (VI) Oxide and Zinc (II) Oxide Nanoparticles Enriched Polyethylene Sebacate Nanocomposites" Polymers 13, no. 5: 718. https://doi.org/10.3390/polym13050718