Combination of Self-Healing Butyl Rubber and Natural Rubber Composites for Improving the Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Self-Healing BIIR/NR Composites
2.3. Characterization
2.3.1. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.3.2. Cure Characteristics
2.3.3. Tensile Properties
2.3.4. Payne Effect
2.3.5. Morphologies
2.3.6. Electrical Conductivity
2.3.7. Abrasion Resistance
2.3.8. Dynamic Mechanical Analysis
3. Results and Discussion
3.1. Interactions of IL and IM with Rubber Molecules
3.2. Cure Characteristics
3.3. Relations of Mechanical, Dynamic Mechanical, and Morphologies
3.3.1. Before Self-Healing Propagation
3.3.2. After Self-Healing Propagation
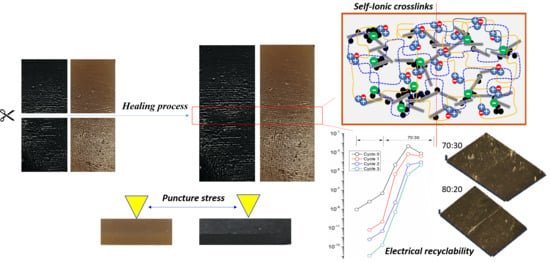
3.4. Durability of the Composites
3.4.1. Relation between Puncture Stress and Electrical Conduction Recyclability
3.4.2. Relation between Abrasion Resistance and Dynamic Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Lu, C.; Zhang, X.; Zhou, Z. Conductive natural rubber/carbon Black nanocomposites via cellulose nanowhisker templated assembly: Tailored hierarchical structure leading to synergistic property enhancements. J. Mater. Chem. A 2015, 25, 13317–13323. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Leung, C.; Hu, R.; Zhao, X.; Yan, B.; Zhou, J. Effect of styrene-butadiene rubber on the electrical properties of carbon black/cement mortar. RSC Adv. 2015, 86, 70229–70237. [Google Scholar] [CrossRef]
- Ye, Q.; Zheng, P.; Ao, X.; Yao, D.; Lei, Z.; Deng, Y.; Wang, C. Novel multi-block conductive binder with polybutadiene for Si anodes in lithium-ion batteries. Electro Acta 2019, 315, 58–66. [Google Scholar] [CrossRef]
- Bernal-Ortega, P.; Bernal, M.M.; González-Jiménez, A.; Posadas, P.; Navarro, R.; Valentín, J.L. New insight into structure-property relationships of natural rubber and styrene-butadiene rubber nanocomposites filled with MWCNT. Polymer 2020, 201, 1–10. [Google Scholar] [CrossRef]
- Malas, A.; Pal, P.; Das, C.K. Effect of expanded graphite and modified graphite flakes on the physical and thermo-mechanical properties of styrene butadiene rubber/polybutadiene rubber (SBR/BR) blends. Mater. Des. 2014, 55, 664–673. [Google Scholar] [CrossRef]
- Le, H.H.; Parsaker, M.; Sriharish, M.N.; Henning, S.; Menzel, M.; Wiessner, S.; Das, A.; Do, Q.K.; Heinrich, G.; Radusch, H. Effect of rubber polarity on selective wetting of carbon nanotubes in ternary blends. Express. Polym. Lett. 2015, 11, 960–971. [Google Scholar] [CrossRef]
- Obata, Y.; Kawabata, S.; Kawai, H. Mechanical properties of natural rubber vulcanizates in finite deformation. J. Polym. Sci. B Polym. Phys. 1970, 6, 903–919. [Google Scholar] [CrossRef]
- Kim, M.; Kim, D.; Chowdhury, S.R.; Kim, G. Melt-compounded butadiene rubber nanocomposites with improved mechanical properties and abrasion resistance. J. Appl. Polym. Sci. 2003, 102, 2062–2066. [Google Scholar] [CrossRef]
- Sadhu, S.; Bhowmick, A. Preparation and characterization of styrene butadiene rubber based Nanocomposites and study of their mechanical properties. Adv. Eng. Mater. 2004, 9, 738–742. [Google Scholar] [CrossRef]
- Habibollah, B.; Ghasem, N.; Sedigheh, S. SBR composites reinforced with N-isopropyl-N′-phenyl-P-phenylenediamine-modified clay. Chin. J. Polym. Sci. 2011, 29, 191–196. [Google Scholar]
- Mehdi, M.; Peyman, G.B.; Ehsan, N. A facile method for dye and heavy metal elimination by pH sensitive acid activated montmorillonite/polyethersulfone nanocomposite membrane. Chin. J. Polym. Sci. 2018, 36, 49–57. [Google Scholar]
- Ye, L.; Ren, J.; Cai, S.Y.; Wang, Z.G.; Li, J.B. Poly(lactic acid) nanocomposites with improved flame retardancy and impact strength by combining of phosphinates and organoclay. Chin. J. Polym. Sci. 2016, 34, 785–796. [Google Scholar]
- Chen, L.; Jia, Z.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci. Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- Zare, Y. Assumption of interphase properties in classical Christensen–Lo model for Young’s modulus of polymer nanocomposites reinforced with spherical nanoparticles. RSC Adv. 2015, 5, 95532–95538. [Google Scholar] [CrossRef]
- Matchawet, S. Nakason, C. Kaesaman, A. Electrical and mechanical properties of conductive carbon black filled epoxidized natural rubber. Adv. Mater. Res. 2013, 844, 255–258. [Google Scholar] [CrossRef]
- Ma, X.; Zare, Y.; Rhee, K.Y. A two-step methodology to study the influence of aggregation/agglomeration of nanoparticles on Young’s modulus of polymer nanocomposites. Nanoscale Res. Lett. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Development of Hashin-Shtrikman model to determine the roles and properties of interphases in clay/CaCO3/PP ternary nanocomposite. Appl. Clay Sci. 2017, 137, 176–182. [Google Scholar] [CrossRef]
- Ma, W.L.; Cai, Z.H.; Zhang, Y.; Zi-Yuan, W.; Lun, X.; Su-Ping, M.; Li, G.H.; Huang, Y. An overview of stretchable supercapacitors based on carbon nanotube and graphene. Chin. J. Polym. Sci. 2020, 38, 491–505. [Google Scholar]
- Kong, E.; Yoon, B.; Nam, J.; Suhr, J. Accelerated aging and lifetime prediction of graphene-reinforced natural rubber composites. Macromol. Res. 2018, 26, 998–1003. [Google Scholar] [CrossRef]
- Imtiaz, S.; Siddiq, M.; Kausar, A.; Muntha, S.T.; Ambreen, J.; Bibi, I. A Review featuring fabrication, properties and applications of carbon nanotubes (CNTs) reinforced polymer and epoxy nanocomposites. Chin. J. Polym. Sci. 2018, 36, 445–461. [Google Scholar] [CrossRef]
- Jian, M.; Zhang, Y.; Liu, Z. Natural biopolymers for flexible sensing and energy devices. Chin. J. Polym. Sci. 2020, 38, 459–490. [Google Scholar] [CrossRef]
- Kummerlöwe, C.; Vennemann, N.; Pieper, S.; Siebert, A.; Nakaramontri, Y. Preparation and properties of carbon-nanotube composites with natural rubber and epoxidized natural rubber. Polimery 2018, 59, 811–818. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Pichaiyut, S.; Wisunthorn, S.; Nakason, C. Hybrid carbon nanotubes and conductive carbon black in natural rubber composites to enhance electrical conductivity by reducing gaps separating carbon nanotube encapsulates. Eur. Polym. J. 2017, 90, 467–484. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Modeling of viscosity and complex modulus for poly (lactic acid)/poly (ethylene oxide)/carbon nanotubes nanocomposites assuming yield stress and network breaking time. Compos. B. Eng. 2019, 156, 100–107. [Google Scholar] [CrossRef]
- Abrisham, M.; Panahi-Sarmad, M.; Sadeghi, G.M.M.; Arjmand, M.; Dehghan, P.; Amirkiai, A. Microstructural design for enhanced mechanical property and shape memory behavior of polyurethane nanocomposites: Role of carbon nanotube, montmorillonite, and their hybrid fillers. Polym. Test. 2020, 89, 1–10. [Google Scholar] [CrossRef]
- Ahmed, S.; Neil, J.C. The synthesis, properties and uses of carbon materials with helical morphology. J. Adv. Res. 2012, 3, 195–223. [Google Scholar]
- Wang, J.J.; Zhang, Q.; Ji, X.X.; Li-Bin, L. Highly stretchable, compressible, adhesive, conductive self-healing composite hydrogels with sensor capacity. Chin. J. Polym. Sci. 2020, 38, 1221–1229. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Development of a conventional model to predict the electrical conductivity of polymer/carbon nanotubes nanocomposites by interphase, waviness and contact effects. Compos. Part A Appl. Sci. Manuf. 2017, 100, 305–312. [Google Scholar]
- Nakaramontri, Y.; Kummerlöwe, C.; Nakason, C.; Vennemann, N. The effect of surface functionalization of carbon nanotubes on properties of natural rubber/carbon nanotube composites. Polym. Compos. 2014, 36, 2113–2122. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Nakason, C.; Kummerlöwe, C.; Vennemann, N. Influence of modified natural rubber on properties of natural rubber–carbon nanotube composites. Rubber. Chem. Technol. 2015, 88, 199–218. [Google Scholar] [CrossRef]
- Sun, Y.; Bao, H.; Guo, Z.; Yu, J. Modeling of the electrical percolation of mixed carbon fillers in polymer-based composites. Macromolecules 2009, 42, 459–463. [Google Scholar] [CrossRef]
- Zhang, S.M.; Lin, L.; Deng, H.; Gao, X.; Bilotti, E.; Peijs, T.; Fu, Q. Synergistic effect in conductive networks constructed with carbon nanofillers in different dimensions. Express Polym. Lett. 2012, 6, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Sallat, A.; Böhme, F.; Suckow, M.; Basu, D.; Wießner, S.; Heinrich, G. Ionic modification turns commercial rubber into a self-healing material. ACS Appl. Mater. Interfaces 2015, 7, 20623–20630. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Hait, S.; Das, A.; Wiessner, S.; Stoeckelhuber, S.; Boehme, K.W.F. Radusch, H. Self-healing properties of carbon nanotube filled natural rubber/bromobutyl rubber blend. Express Polym. Lett. 2017, 11, 230–242. [Google Scholar] [CrossRef]
- Khalil, A.; Shaikh, S.; Nizami, N.; Nudrat, Z.R. Reinforcement of natural rubber hybrid composites based on marble sludge/Silica and marble sludge/rice husk derived silica. J. Adv. Res. 2014, 5, 165–173. [Google Scholar]
- Zheng, P.; Chunfang, F.; Yongyue, L.; Yongzhen, L.; Kong, L.X. Self-assembled natural rubber/multi-walled carbon nanotube composites using latex compounding techniques. Carbon 2010, 48, 4497–4503. [Google Scholar]
- Elbadawy, A.K.; El-Refaie, S.K.; Xin, C. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar]
- Rajkumar, T.; Ranga, G.R. Synthesis and characterization of hybrid molecular material prepared by Ionic liquid and silicotungstic acid. Mater. Chem. Phys. 2008, 112, 853–857. [Google Scholar] [CrossRef]
- Makled, M.H.; Sheha, E.; Shanap, T.S.; El-Mansy, M.K. Electrical conduction and dielectric relaxation in p-type PVA/CuI polymer composite. J. Adv. Res. 2013, 4, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Le, H.H.; Wießner, S.; Das, A.; Fischer, D.; Auf der Landwehr, M.; Do, Q.; Radusch, H. Selective wetting of carbon nanotubes in rubber compounds—Effect of the Ionic liquid as dispersing and coupling agent. Eur. Polym. J. 2016, 75, 13–24. [Google Scholar] [CrossRef]
- Galiani, P.D.; Antonio, J.M.; Guenther, B.S.; Henrique, L.C.M. Studies on thermal–oxidative degradation behaviours of raw natural rubber: PRI and thermogravimetry analysis. Plast. Rub. Compos. 2013, 42, 334–339. [Google Scholar] [CrossRef]
- Kruželák, J.; Hudec, I. Vulcanization systems for rubber compounds based on IIR and halogenated IIR: An overview. Rubber. Chem. Technol. 2018, 91, 167–183. [Google Scholar] [CrossRef]
- Tunckol, M.; Durand, J.; Serp, P. Carbon nanomaterial–Ionic liquid hybrids. Carbon 2012, 50, 4303–4334. [Google Scholar] [CrossRef]
- Bolesław, S.; Anna, M.; Marian, Z. Use of carbon black as a reinforcing nano-filler in conductivity-reversible elastomer composites. Polym. Test. 2020, 81, 106222. [Google Scholar]
- Matchawet, S.; Kaesamana, A.; Vennemann, N.; Kumerlöwe, C.; Nakason, C. Effects of imidazolium ionic liquid on cure characteristics, electrical conductivity and other related properties of epoxidized natural rubber vulcanizates. Eur. Polym. J. 2017, 37, 344–359. [Google Scholar] [CrossRef]
- Krainoia, A.; Nakaramontri, Y.; Kummerlöwe, C.; Vennemann, N.; Wisunthorn, S.; Pichaiyut, S.; Nakason, C. Influence of carbon nanotube and ionic liquid on properties of natural rubber nanocomposites. J. Appl. Polym. Sci. 2019, 13, 327–348. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. The mechanical behavior of CNT reinforced nanocomposites assuming imperfect interfacial bonding between matrix and nanoparticles and percolation of interphase regions. Compos. Sci. Technol. 2017, 144, 18–25. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Tensile strength prediction of carbon nanotube reinforced composites by expansion of cross-orthogonal skeleton structure. Compos. B Eng. 2019, 161, 601–607. [Google Scholar]
- Zare, Y.; Rhee, K.Y. A simple methodology to predict the tunneling conductivity of polymer/CNT nanocomposites by the roles of tunneling distance, interphase and CNT waviness. RSC Adv. 2017, 7, 34912–34921. [Google Scholar]
- Heinz, M.; Grosch, K.A. A Laboratory method to comprehensively evaluate abrasion, traction and rolling resistance of tire tread compounds. Rubber Chem. Technol. 2007, 80, 580–607. [Google Scholar] [CrossRef]
- Le, H.H.; Böhme, F.; Sallat, A.; Wießner, S.; Auf der Landwehr, M.; Reuter, U.; Das, A. Triggering the self-healing properties of modified bromobutyl rubber by intrinsically electrical heating. Macromol. Mater. Eng. 2016, 302, 1600385. [Google Scholar] [CrossRef]
- Sattayanurak, S.; Sahakaro, K.; Kaewsakul, W.; Dierkes, W.K.; Reuvekamp, L.A.; Blume, A.; Noordermeer, J.W. Synergistic effect by high specific surface area carbon black as secondary filler in silica reinforced natural rubber tire tread compounds. Polym. Test. 2020, 81, 106173. [Google Scholar] [CrossRef]
















| Chemicals | Contents (phr*) | |
|---|---|---|
| Modification of BIIR (COMPOUND I) | ||
| BIIR | 100 | 70 or 80 |
| IL or IM | 5 and 10 | |
| Preparation of NR composites (COMPOUND II) | ||
| NR | 100 | 30 or 20 |
| CNT:CB | 5:7.5 | |
| ZnO | 5 | |
| Stearic acid | 1 | |
| MBTS | 1 | |
| Sulfur | 2.5 | |
| Samples | ML (d.Nm) | MH (d.Nm) | T90 (min) | TS1 (min) | MH–ML (d.Nm) |
|---|---|---|---|---|---|
| Pure BIIR | 1.14 | 1.40 | − | − | 0.27 |
| Pure NR | 1.07 | 5.39 | 3.28 | 1.28 | 4.32 |
| BIIR:NR ratio = 70:30 | |||||
| BIIR/NR | 0.73 | 2.19 | 23.85 | 19.07 | 1.45 |
| BIIR/NR-CNTCB | 1.07 | 3.19 | 21.26 | 8.75 | 2.12 |
| BIIR/NR-CNTCB-IL5 | 1.12 | 3.28 | 5.53 | 1.62 | 2.16 |
| BIIR/NR-CNTCB-IM5 | 1.26 | 2.30 | 3.28 | 4.25 | 1.04 |
| BIIR:NR ratio = 80:20 | |||||
| BIIR/NR | 0.86 | 2.09 | 23.55 | 21.58 | 1.24 |
| BIIR/NR-CNTCB | 1.07 | 2.68 | 21.74 | 16.40 | 1.62 |
| BIIR/NR-CNTCB-IL5 | 1.15 | 2.61 | 10.25 | 4.04 | 1.45 |
| BIIR/NR-CNTCB-IM5 | 1.32 | 1.72 | 2.52 | − | 0.39 |
| Samples | 100 % Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| Pure NR | 1.31 ± 0.02 | 14.50 ± 0.15 | 1412.45 ± 5.34 |
| Pure BIIR | 0.36 ± 0.05 | 0.12 ± 0.10 | 3124.45 ± 6.55 |
| BIIR:NR ratio = 70:30 | |||
| BIIR/NR | 0.35 ± 0.04 | 2.32 ± 0.12 | 1504.17 ± 14.56 |
| BIIR/NR-CNTCB | 0.48 ± 0.11 | 4.47 ± 0.25 | 1164.04 ± 23.16 |
| BIIR/NR-CNTCB-IL5 | 0.96 ± 0.10 | 3.75 ± 0.10 | 551.82 ± 16.65 |
| BIIR/NR-CNTCB-IM5 | 0.50 ± 0.06 | 3.31 ± 0.22 | 1743.16 ± 34.55 |
| BIIR:NR ratio = 80:20 | |||
| BIIR/NR | 0.35 ± 0.05 | 1.57 ± 0.15 | 1159.44 ± 21.76 |
| BIIR/NR-CNTCB | 0.44 ± 0.01 | 2.45 ± 0.51 | 1064.14 ± 62.98 |
| BIIR/NR-CNTCB-IL5 | 0.70 ± 0.02 | 2.82 ± 0.32 | 564.75 ± 44.23 |
| BIIR/NR-CNTCB-IM5 | 0.40 ± 0.04 | 1.79 ± 0.57 | 2476.55 ± 24.45 |
| Samples | 100 % Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| Pure NR | − | − | − |
| Pure BIIR | 0.33 ± 0.12 | 0.27 ± 0.15 | 674.65 ± 23.34 |
| BIIR:NR ratio = 70:30 | |||
| BIIR/NR | 0.34 ± 0.05 | 0.48 ± 0.12 | 396.59 ± 11.33 |
| BIIR/NR-CNTCB | 0.42 ± 0.06 | 0.51 ± 0.11 | 257.80 ± 32.23 |
| BIIR/NR-CNTCB-IL5 | 0.50 ± 0.01 | 0.50 ± 0.08 | 166.10 ± 14.43 |
| BIIR/NR-CNTCB-IM5 | 0.48 ± 0.14 | 0.78 ± 0.19 | 497.80 ± 15.18 |
| BIIR:NR ratio = 80:20 | |||
| BIIR/NR | 0.33 ± 0.04 | 0.46 ± 0.02 | 336.73 ± 32.33 |
| BIIR/NR-CNTCB | 0.41 ± 0.16 | 0.47 ± 0.05 | 283.77 ± 16.43 |
| BIIR/NR-CNTCB-IL5 | 0.53 ± 0.07 | 0.54 ± 0.23 | 191.13 ± 18.55 |
| BIIR/NR-CNTCB-IM5 | 0.50 ± 0.12 | 0.68 ± 0.45 | 502.25 ± 25.78 |
| Samples | Tg (°C) | Tan δmax | Tan δ0 | Tan δ60 |
|---|---|---|---|---|
| Pure NR | −52.6 | 2.03 | 0.20 | 0.04 |
| Pure BIIR | −33.0 | 1.45 | 0.49 | 0.20 |
| BIIR:NR ratio = 70:30 | ||||
| BIIR/NR | −36.1 | 1.41 | 0.35 | 0.13 |
| BIIR/NR-CNTCB | −36.6 | 1.27 | 0.36 | 0.12 |
| BIIR/NR-CNTCB-IL5 | −36.6 | 1.28 | 0.26 | 0.10 |
| BIIR/NR-CNTCB-IM5 | −36.4 | 1.25 | 0.37 | 0.11 |
| BIIR:NR ratio = 80:20 | ||||
| BIIR/NR | −35.6 | 1.43 | 0.36 | 0.14 |
| BIIR/NR-CNTCB | −36.0 | 1.33 | 0.41 | 0.13 |
| BIIR/NR-CNTCB-IL5 | −36.1 | 1.36 | 0.31 | 0.10 |
| BIIR/NR-CNTCB-IM5 | −5.7 | 1.32 | 0.43 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumnum, K.; Kalkornsurapranee, E.; Johns, J.; Sengloyluan, K.; Nakaramontri, Y. Combination of Self-Healing Butyl Rubber and Natural Rubber Composites for Improving the Stability. Polymers 2021, 13, 443. https://doi.org/10.3390/polym13030443
Chumnum K, Kalkornsurapranee E, Johns J, Sengloyluan K, Nakaramontri Y. Combination of Self-Healing Butyl Rubber and Natural Rubber Composites for Improving the Stability. Polymers. 2021; 13(3):443. https://doi.org/10.3390/polym13030443
Chicago/Turabian StyleChumnum, Kunakorn, Ekwipoo Kalkornsurapranee, Jobish Johns, Karnda Sengloyluan, and Yeampon Nakaramontri. 2021. "Combination of Self-Healing Butyl Rubber and Natural Rubber Composites for Improving the Stability" Polymers 13, no. 3: 443. https://doi.org/10.3390/polym13030443