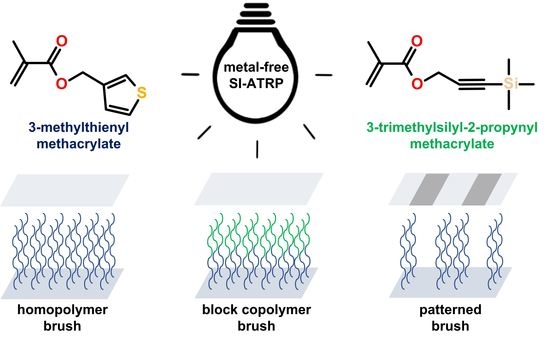
Preparation of Homopolymer, Block Copolymer, and Patterned Brushes Bearing Thiophene and Acetylene Groups Using Microliter Volumes of Reaction Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Procedures
2.3.1. Surface Cleaning and Immobilization of the Initiator
2.3.2. Surface-Initiated Atom Transfer Radical Polymerization (SI-ATRP) of 3-Methylthienyl Methacrylate (MTM) and 3-Trimethylsilyl-2-propynyl Methacrylate (TPM)
2.3.3. Metal-Free Surface-Initiated Atom Transfer Radical Polymerization (Metal-Free SI-ATRP) of MTM and TPM
2.3.4. Chain Extension Experiment
2.3.5. Synthesis of ITO-g-PMTM-b-PTPM and ITO-g-PTPM-b-PMTM Block Copolymer Brushes by Metal-Free SI-ATRP
2.3.6. Self-Templating Polymerization of ITO-g-PMTM-co-PTPM
2.3.7. Synthesis of Patterned Polymer Brushes by Metal-Free SI-ATRP
3. Results
3.1. Copper-Based SI-ATRP
3.2. Metal-Free SI-ATRP of MTM and TPM Monomers
3.3. Chain Extension and Formation of Block Copolymer Brushes
3.4. Polymer Chain Conjugation
3.5. Spatially Controlled Decoration of Inorganic Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Bockstaller, M.R.; Matyjaszewski, K. Brush-modified materials: Control of molecular architecture, assembly behavior, properties and applications. Prog. Polym. Sci. 2020, 100, 101180. [Google Scholar] [CrossRef]
- Gruszkiewicz, A.; Słowikowska, M.; Grześ, G.; Wójcik, A.; Rokita, J.; Fiocco, A.; Wytrwal-Sarna, M.; Marzec, M.; Trzebicka, B.; Kopeć, M.; et al. Enhancement of the growth of polymer brushes via ATRP initiated from ions-releasing indium tin oxide substrates. Eur. Polym. J. 2019, 112, 817–821. [Google Scholar] [CrossRef]
- Poręba, R.; de los Santos Pereira, A.; Pola, R.; Jiang, S.; Pop-Georgievski, O.; Sedláková, Z.; Schönherr, H. “Clickable” and Antifouling Block Copolymer Brushes as a Versatile Platform for Peptide-Specific Cell Attachment. Macromol. Biosci. 2020, 20, 1900354. [Google Scholar] [CrossRef] [Green Version]
- Mocny, P.; Menétrey, M.; Klok, H.A. Synthesis of Loop Poly(Methyl Methacrylate) Brushes via Chain-End Postpolymerization Modification. Macromolecules 2019, 52, 8394–8403. [Google Scholar] [CrossRef]
- Raj, W.; Russo, A.; Zhang, Y.; Chapelat, J.; Pietrasik, J. Renewable fabric surface-initiated ATRP polymerizations: Towards mixed polymer brushes. Nanomaterials 2020, 10, 536. [Google Scholar] [CrossRef] [Green Version]
- Wolski, K.; Gruszkiewicz, A.; Wytrwal-Sarna, M.; Bernasik, A.; Zapotoczny, S. The grafting density and thickness of polythiophene-based brushes determine the orientation, conjugation length and stability of the grafted chains. Polym. Chem. 2017, 8, 6250–6262. [Google Scholar] [CrossRef]
- Kopeć, M.; Pikiel, M.; Vancso, G.J. Surface-grafted polyacrylonitrile brushes with aggregation-induced emission properties. Polym. Chem. 2020, 11, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Flejszar, M.; Chmielarz, P.; Wolski, K.; Grześ, G.; Zapotoczny, S. Polymer Brushes via Surface-Initiated Electrochemically Mediated ATRP: Role of a Sacrificial Initiator in Polymerization of Acrylates on Silicon Substrates. Materials 2020, 13, 3559. [Google Scholar] [CrossRef]
- Faggion Albers, R.; Yan, W.; Romio, M.; Leite, E.R.; Spencer, N.D.; Matyjaszewski, K.; Benetti, E.M. Mechanism and application of surface-initiated ATRP in the presence of a Zn0 plate. Polym. Chem. 2020, 11, 7009–7014. [Google Scholar] [CrossRef]
- Poelma, J.E.; Fors, B.P.; Meyers, G.F.; Kramer, J.W.; Hawker, C.J. Fabrication of complex three-dimensional polymer brush nanostructures through light-mediated living radical polymerization. Angew. Chemie 2013, 52, 6844–6848. [Google Scholar] [CrossRef]
- Słowikowska, M.; Chajec, K.; Michalski, A.; Zapotoczny, S.; Wolski, K. Surface-Initiated Photoinduced Iron-Catalyzed Atom Transfer Radical Polymerization with ppm Concentration of FeBr3 under Visible Light. Materials 2020, 13, 5139. [Google Scholar] [CrossRef]
- Yan, W.; Dadashi-Silab, S.; Matyjaszewski, K.; Spencer, N.D.; Benetti, E.M. Surface-Initiated Photoinduced ATRP: Mechanism, Oxygen Tolerance, and Temporal Control during the Synthesis of Polymer Brushes. Macromolecules 2020, 53, 2801–2810. [Google Scholar] [CrossRef]
- Zhao, H.; Sha, J.; Wang, X.; Jiang, Y.; Chen, T.; Wu, T.; Chen, X.; Ji, H.; Gao, Y.; Xie, L.; et al. Spatiotemporal control of polymer brush formation through photoinduced radical polymerization regulated by DMD light modulation. Lab Chip 2019, 19, 2651–2662. [Google Scholar] [CrossRef]
- Ballav, N.; Schilp, S.; Zharnikov, M. Electron-beam chemical lithography with aliphatic self-assembled monolayers. Angew. Chemie 2008, 47, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, Z.; Gan, T.; Wang, Y.; Zhang, G.; Mirkin, C.A.; Zheng, Z. Biomimicking Nano-Micro Binary Polymer Brushes for Smart Cell Orientation and Adhesion Control. Small 2016, 12, 3400–3406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Z.; Xie, Z.; Liu, X.; Zheng, Z. High-resolution, large-area, serial fabrication of 3D polymer brush structures by parallel dip-pen nanodisplacement lithography. Small 2012, 8, 3568–3572. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.R.; Cheng, C.C.; Lee, A.W.; Wei, P.L.; Chen, J.K. Visualization platform of one-dimensional gratings of tethered polyvinyltetrazole brushes on silicon surfaces for sensing of Cr(III). Microchim. Acta 2017, 184, 2723–2730. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Lee, A.W.; Chang, J.Y.; Huang, C.H.; Chen, J.K. Fabrication of metamaterial absorber using polymer brush-gold nanoassemblies for visualizing the reversible pH-responsiveness. J. Mater. Chem. C 2014, 2, 8226–8234. [Google Scholar] [CrossRef]
- Lamping, S.; Stricker, L.; Ravoo, B.J. Responsive surface adhesion based on host-guest interaction of polymer brushes with cyclodextrins and arylazopyrazoles. Polym. Chem. 2019, 10, 683–690. [Google Scholar] [CrossRef]
- Yu, Q.; Ista, L.K.; Gu, R.; Zauscher, S.; López, G.P. Nanopatterned polymer brushes: Conformation, fabrication and applications. Nanoscale 2016, 8, 680–700. [Google Scholar] [CrossRef]
- Welch, M.E.; Ober, C.K. Responsive and patterned polymer brushes. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1457–1472. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, X.; Matyjaszewski, K. Atom Transfer Radical Polymerization of 4-Vinylpyridine. Macromolecules 1999, 32, 3531–3533. [Google Scholar] [CrossRef]
- Discekici, E.H.; Pester, C.W.; Treat, N.J.; Lawrence, J.; Mattson, K.M.; Narupai, B.; Toumayan, E.P.; Luo, Y.; McGrath, A.J.; Clark, P.G.; et al. Simple Benchtop Approach to Polymer Brush Nanostructures Using Visible-Light-Mediated Metal-Free Atom Transfer Radical Polymerization. ACS Macro Lett. 2016, 5, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Narupai, B.; Page, Z.A.; Treat, N.J.; McGrath, A.J.; Pester, C.W.; Discekici, E.H.; Dolinski, N.D.; Meyers, G.F.; Read de Alaniz, J.; Hawker, C.J. Simultaneous Preparation of Multiple Polymer Brushes under Ambient Conditions using Microliter Volumes. Angew. Chemie 2018, 57, 13433–13438. [Google Scholar] [CrossRef] [PubMed]
- Słowikowska, M.; Wójcik, A.J.; Wolski, K.; Hatalak, A.; Zapotoczny, S. Light-promoted synthesis of surface-grafted polymers bearing pyridine groups by metal-free ATRP in microliter volumes. Polymer 2021, 234, 124244. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Wolski, K.; Kruk, T.; Zapotoczny, S. Macromolecular strategies for transporting electrons and excitation energy in ordered polymer layers. Prog. Polym. Sci. 2021, 121, 101433. [Google Scholar] [CrossRef]
- Yang, L.; Sontag, S.K.; Lajoie, T.W.; Li, W.; Huddleston, N.E.; Locklin, J.; You, W. Surface-initiated poly(3-methylthiophene) as a hole-transport layer for polymer solar cells with high performance. ACS Appl. Mater. Interfaces 2012, 4, 5069–5073. [Google Scholar] [CrossRef]
- Tria, M.C.; Liao, K.S.; Alley, N.; Curran, S.; Advincula, R. Electrochemically crosslinked surface-grafted PVK polymer brushes as a hole transport layer for organic photovoltaics. J. Mater. Chem. 2011, 21, 10261–10264. [Google Scholar] [CrossRef]
- Tkachov, R.; Senkovskyy, V.; Oertel, U.; Synytska, A.; Horecha, M.; Kiriy, A. Microparticle-supported conjugated polyelectrolyte brushes prepared by surface-initiated Kumada catalyst transfer polycondensation for sensor applications. Macromol. Rapid Commun. 2010, 31, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, A.J.; Wolski, K.; Zapotoczny, S. Double-stranded surface-grafted polymer brushes with ladder-like architecture. Eur. Polym. J. 2021, 155, 110577. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Wolski, K.; Pomorska, A.; Uchacz, T.; Gut, A.; Łapok, Ł.; Zapotoczny, S. Photoactive Surface-Grafted Polymer Brushes with Phthalocyanine Bridging Groups as an Advanced Architecture for Light-Harvesting. Chem. Eur. J. 2017, 23, 11239–11243. [Google Scholar] [CrossRef] [PubMed]
- Słowikowska, M.; Wolski, K.; Wójcik, A.J.; Wesner, D.; Schönherr, H.; Zapotoczny, S. Unraveling the nanomechanical properties of surface-grafted conjugated polymer brushes with ladder-like architecture. Polym. Chem. 2020, 11, 7050–7062. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Wolski, K.; Zapotoczny, S. Enhanced stability of conductive polyacetylene in ladder-like surface-grafted brushes. Polym. Chem. 2016, 7, 5664–5670. [Google Scholar] [CrossRef]
- Wolski, K.; Gruszkiewicz, A.; Zapotoczny, S. Conductive polythiophene-based brushes grafted from an ITO surface via a self-templating approach. Polym. Chem. 2015, 6, 7505–7513. [Google Scholar] [CrossRef]
- Wolski, K.; Szuwarzyński, M.; Zapotoczny, S. A facile route to electronically conductive polyelectrolyte brushes as platforms of molecular wires. Chem. Sci. 2015, 6, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çirpan, A.; Alkan, S.; Toppare, L.; Hepuzer, Y.; Yaǧci, Y. Conducting graft copolymers of poly(3-methylthienyl methacrylate) with pyrrole and thiophene. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4131–4140. [Google Scholar] [CrossRef]
- Saha, S.; Baker, G.L. Surface-tethered conjugated polymers created via the grafting-from approach. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Treat, N.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G.; Barton, B.E.; Read De Alaniz, J.; Fors, B.P.; Hawker, C.J. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef] [Green Version]
- Kelkar, D.; Chourasia, A. Structural properties of polythiophene doped with FeCl3. Chem. Chem. Technol. 2011, 5, 309–315. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smenda, J.; Wolski, K.; Chajec, K.; Zapotoczny, S. Preparation of Homopolymer, Block Copolymer, and Patterned Brushes Bearing Thiophene and Acetylene Groups Using Microliter Volumes of Reaction Mixtures. Polymers 2021, 13, 4458. https://doi.org/10.3390/polym13244458
Smenda J, Wolski K, Chajec K, Zapotoczny S. Preparation of Homopolymer, Block Copolymer, and Patterned Brushes Bearing Thiophene and Acetylene Groups Using Microliter Volumes of Reaction Mixtures. Polymers. 2021; 13(24):4458. https://doi.org/10.3390/polym13244458
Chicago/Turabian StyleSmenda, Joanna, Karol Wolski, Kamila Chajec, and Szczepan Zapotoczny. 2021. "Preparation of Homopolymer, Block Copolymer, and Patterned Brushes Bearing Thiophene and Acetylene Groups Using Microliter Volumes of Reaction Mixtures" Polymers 13, no. 24: 4458. https://doi.org/10.3390/polym13244458