An Overview of Recent Advancements in Microbial Polyhydroxyalkanoates (PHA) Production from Dark Fermentation Acidogenic Effluents: A Path to an Integrated Bio-Refinery
Abstract
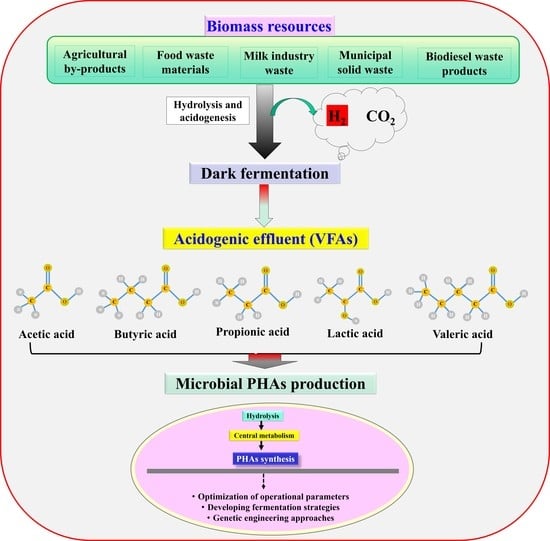
:1. Introduction
2. Chemical Properties and Market Potential of Acidogenic Effluent (VFAs)
3. Importance of PHA Relative to Synthetic Plastics
4. VFAs as a Potential and Inexpensive Substrate for PHA Production
5. Metabolic Pathways Using Various VFAs
6. Approaches to Enhance VFAs to PHA Synthesis
6.1. Optimization of Fermentation Process Parameters
6.2. Employing Various Fermentation Mode and Feeding Strategies
6.3. Using Mixed Microbial Culture
6.4. Genetic Engineering Approaches for Sustainable PHA Production Using VFAs
7. Conclusions
- (1)
- Optimization of process parameters to generate VFAs in good quantity in the fermented waste. More research is needed for the cost effective separation of VFAs from acidogenic effluents. A further obstacle is the presence of ammonium and phosphorous in the VFA-rich acidogenic effluents.
- (2)
- Extensive research and development is needed for the isolation of effective microbial strains which can tolerate VFAs and can effectively convert them into PHA with a high production rate.
- (3)
- A major focus should be the effective downstream processing for the isolation of PHA with higher productivity and purity. The process should be cost effective and eco-friendly by avoiding input of harsh chemicals.
- (4)
- It is a necessity to develop effective microbial strains by applying genetic engineering and molecular tools that can lead to efficient VFAs utilisation, enhanced biomass yield, and PHA productivity.
- (5)
- Significant attention should be devoted to PHA studies at pilot and commercial scales to understand the molecular and engineering issues.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leong, H.Y.; Chang, C.K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.S.; Show, P.L. Waste biorefinery towards a sustainable circular bioeconomy: A solution to global issues. Biotechnol. Biofuels 2021, 14, 87. [Google Scholar] [CrossRef]
- Srivastava, N.; Hussain, A.; Kushwaha, D.; Haque, S.; Mishra, P.K.; Gupta, V.K.; Srivastava, M. Nickel ferrite nanoparticles induced improved fungal cellulase production using residual algal biomass and subsequent hydrogen production following dark fermentation. Fuel 2021, 304, 121391. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kuppam, C.; Mudhoo, A.; Saratale, G.D.; Kadier, A.; Zhen, G.; Chatellard, L.; Trably, E.; Kumar, G. A comprehensive review on two-stage integrative schemes for the valorization of dark fermentative effluents. Crit. Rev. Biotechnol. 2018, 38, 868–882. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Banu, J.R.; Chang, J.S. Biohydrogen production from renewable biomass resources. Biohydrogen (second edition). Biomass Biofuels Biochem. 2019, 247–277. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.-H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Eljack, F.; Kazi, M.-K. Prospects and Challenges of Green Hydrogen Economy via Multi-Sector Global Symbiosis in Qatar. Front. Sustain. 2021, 1, 612762. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Mudhoo, A.; Rene, E.R.; Saratale, G.D.; Kobayashi, T.; Xu, K.; Kim, S.H.; Kim, D.H. Fermentative hydrogen production using lignocellulose biomass: An overview of pre-treatment methods, inhibitor effects and detoxification experiences. Renew. Sustain. Energy Rev. 2017, 77, 28–42. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Chandrasekhar, K.; Mohanakrishna, G.; Saratale, G.D.; Saratale, R.G.; Kumar, G.; Pugazhendhi, A.; Sivagurunathan, P. Surpassing the current limitations of high purity H2 production in microbial electrolysis cell (MECs): Strategies for inhibiting growth of methanogens. Bioelectrochemistry 2018, 119, 211–219. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ghimire, A.; Ezeokoli, O.T.; Rao, S.; Ngan, W.Y.; Habimana, O.; Yao, Y.; Yang, P.; Fung, A.H.Y.; Yoro, K.O.; et al. Valorization of volatile fatty acids from the dark fermentation waste Streams-A promising pathway for a biorefinery concept. Renew. Sustain. Energy Rev. 2021, 143, 110971. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pilli, S.; Bhunia, P.; Tyagi, R.D.; Surampalli, R.Y.; Zhang, T.C.; Kim, S.H.; Pandey, A. Dark fermentation: Production and utilization of volatile fatty acid from different wastes—A review. Chemosphere 2022, 288, 132444. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Murto, M.; Read, J.S.; Mattiasson, B. Volatile fatty acid production during anaerobic mesophilic digestion of solid potato waste. J. Chem. Technol. Biotechnol. 2004, 79, 673–677. [Google Scholar] [CrossRef]
- Baumann, I.; Westermann, P. Microbial production of short chain fatty acids from lignocellulosic biomass: Current processes and market. BioMed Res. Int. 2016, 2016, 8469357. [Google Scholar] [CrossRef] [Green Version]
- Njokweni, S.G.; Steyn, A.; Botes, M.; Viljoen-Bloom, M.; van Zyl, W.H. Potential Valorization of Organic Waste Streams to Valuable Organic Acids through Microbial Conversion: A South African Case Study. Catalysts 2021, 11, 964. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top. Value-Added Chemicals from Biomass Volume 1—Results of Screening for Potential Candidates from Sugars and Synthesis Gas. (No. DOE/GO-102004-1992); National Renewable Energy Laboratory: Golden, CO, USA, 2004. [Google Scholar]
- Ravindran, A.; Adav, S.; Yang, S.S. Effect of heat pre-treatment temperature on isolation of hydrogen producing functional consortium from soil. Renew. Energy 2010, 35, 2649–2655. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Kutty, P.; Pachapur, P.; Brar, S.K.; Le Bihan, Y.; Galvez-Cloutier, R.; Buelna, G. Seed pretreatment for increased hydrogen production using mixed-culture systems with advantages over pure-culture systems. Energies 2019, 12, 530. [Google Scholar] [CrossRef] [Green Version]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Lovitt, R.W. Complex Effluent Streams as a Potential Source of Volatile Fatty Acids. Waste Biomass Valorization 2013, 4, 557–581. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Yang, Y.-H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Bio/Technol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Kosseva, M.R.; Rusbandi, E. Trends in the biomanufacture of polyhydroxyalkanoates with focus on downstream processing. Int. J. Biol. Macromol. 2018, 107, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Kim, D.S.; Ghodake, G.S.; Kadam, A.; Kumar, G.; Bharagava, R.N.; Banu, R.; Shin, H.S. Pretreatment of kenaf (Hibiscus cannabinus L.) biomass feedstock for polyhydroxybutyrate (PHB) production and characterization. Bioresour. Technol. 2019, 282, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Ghodake, G.S.; Bharagava, R.N.; Kim, D.S.; Nair, S.; Shin, H.S. Efficient bioconversion of sugarcane bagasse into polyhydroxybutyrate (PHB) by Lysinibacillus sp. and its characterization. Bioresour. Technol. 2021, 324, 124673. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.-K.; Ghodake, G.S.; Shin, H.-S.; Saratale, G.D.; Park, Y.; Lee, H.-S.; Bharagava, R.N.; Kim, D.-S. Utilization of Noxious Weed Water Hyacinth Biomass as a Potential Feedstock for Biopolymers Production: A Novel Approach. Polymers 2020, 12, 1704. [Google Scholar] [CrossRef] [PubMed]
- Zinn, M.; Amstutz, V.; Hanik, N.; Pott, J.; Utsunomia, C. Grave-to-cradle: The potential of autotrophic bioprocesses in bioplastic production. New Biotechnol. 2018, 44, S64. [Google Scholar] [CrossRef]
- Ye, J.; Huang, W.; Wang, D.; Chen, F.; Yin, J.; Li, T.; Zhang, H.; Chen, G.-Q. PilotScale-up of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Production by Halomonas bluephagenesis via Cell Growth Adapted Optimization. Process. Biotechnol. J. 2018, 13, e1800074. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Kim, S.H.; Kumar, G. Screening and optimization of pretreatments in the preparation of sugarcane bagasse feedstock for biohydrogen production and process optimization. Int. J. Hydrogen Energy 2018, 43, 11470–11483. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, H.; Chang, J.; Sun, J.; Tu, W.; Wang, H. Effect of thermal hydrolysis pretreatment on volatile fatty acids production in sludge acidification and subsequent polyhydroxyalkanoates production. Bioresour. Technol. 2019, 279, 92–100. [Google Scholar] [CrossRef]
- Reddy, M.; Kotamraju, A.; Venkata Mohan, S. Bacterialsynthesis of polyhydroxyalkanoates using dark fermentation effluents: Comparison between pure and enriched mixed cultures. Eng. Life Sci. 2015, 15, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Colombo, B.; Sciarria, T.P.; Reis, M.; Scaglia, B.; Adani, F. Polyhydroxyalkanoates (PHAs) production from fermented cheese whey by using a mixed microbial culture. Bioresour. Technol. 2016, 218, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Shirai, Y.; Nor Aini, A.R.; Hassan, M.A. Semi-Continuous and Continuous Anaerobic Treatment of Palm Oil Mill Effluent for the Production of Organic Acids and Polyhydroxyalkanoates. Res. J. Environ. Sci. 2009, 3, 552–559. [Google Scholar]
- Mohammadi, M.; Hassan, M.A.; Shirai, Y.; Man, H.C.; Ariffin, H.; Yee, L.-N.; Mumtaz, T.; Chong, M.-L.; Phang, L.-Y. Separationand Purification of Polyhydroxyalkanoates from Newly Isolated Comamonas sp. EB172 by Simple Digestion with Sodium Hydroxide. Sep. Sci. Technol. 2012, 47, 534–541. [Google Scholar] [CrossRef]
- Wang, P.; Qiu, Y.-Q.; Chen, X.-T.; Liang, X.-F.; Ren, L.-H. Metabolomicinsights into polyhydroxyalkanoates production by halophilic bacteria with acetic acid as carbon source. Biosci. Biotechnol. Biochem. 2019, 83, 1955–1963. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; De Wever, H. AceticAcid as an Indirect Sink of CO2 for the Synthesis of Polyhydroxyalkanoates (PHA): Comparison with PHA Production Processes Directly Using CO2 as Feedstock. Appl. Sci. 2018, 8, 1416. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, P.; Gibbons, W.; Muthukumarappan, K. Conversion of volatile fatty acids into polyhydroxyalkanoate by Ralstonia eutropha. J. Appl. Microbiol. 2009, 106, 1996–2005. [Google Scholar] [CrossRef]
- Munir, S.; Jamil, N. Polyhydroxyalkanoates (PHA) production in bacterial co-culture using glucose and volatile fatty acids as carbon source. J. Basic Microbiol. 2018, 58, 247–254. [Google Scholar] [CrossRef] [PubMed]
- López, J.C.; Arnáiz, E.; Merchán, L.; Lebrero, R.; Muñoz, R. Biogas-based polyhydroxyalkanoates production by Methylocystis hirsuta: A step further in anaerobic digestion biorefineries. Chem. Eng. J. 2018, 333, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Catalán, A.I.; Malan, A.K.; Ferreira, F.; Gill, P.R.; Batista, S. Propionicacid metabolism and poly-3-hydroxybutyrate-co-3-hydroxyvalerate production by a prpC mutant of Herbaspirillum seropedicae Z69. J. Biotechnol. 2018, 286, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Sawant, S.S.; Kim, B.S. Production of polyhydroxyalkanoates by Ralstonia eutropha from volatile fatty acids. Korean J. Chem. Eng. 2013, 30, 2223–2227. [Google Scholar] [CrossRef]
- Kemavongse, K.; Poonsuk, P.; Upaichit, A.; Pawadee, M. Effectof co-substrate on production of poly-β- hydroxybutyrate (PHB) and copolymer PHBV from newly identified mutant Rhodobacter sphaeroides U7 cultivated under aerobic-dark condition. Songklanakarin J. Sci. Technol. 2007, 29, 1101–1113. [Google Scholar]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.-K.; Ghodake, G.S.; Kadam, A.; Mulla, S.I.; Bharagava, R.N.; Kim, D.-S.; Shin, H.S. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crop. Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Productionof poly(3-hydroxybutyric-co-3-hydroxyvaleric acid) having a high hydroxyvalerate content with valeric acid feeding. J. Ind. Microbiol. Biotechnol. 2007, 34, 457–461. [Google Scholar] [CrossRef]
- Padovani, G.; Carlozzi, P.; Seggiani, M.; Cinelli, P.; Vitolo, S.; Lazzeri, A. PHB-rich biomass and BioH2 production by means of photosynthetic microorganisms. Inst. Res. Inf. Syst. 2016, 49, 55–60. [Google Scholar]
- Rowaihi, I.; Kick, B.; Grötzinger, S.; Burger, C.; Karan, R.; Weuster-Botz, D.; Eppinger, J.; Arold, S. A two-stage biological gas to liquid transfer process to convert carbon dioxide into bioplastic. Bioresour. Technol. Rep. 2018, 1, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Lagoa-Costa, B.; Abubackar, H.N.; Fernandez-Romasanta, M.; Kennes, C.; Veiga, M.C. Integrated bioconversion of syngas into bioethanol and biopolymers. Bioresour. Technol. 2017, 239, 244–249. [Google Scholar] [CrossRef]
- Haywood, G.W.; Anderson, A.J.; Williams, D.R.; Dawes, E.A.; Ewing, D.F. Accumulation of a poly(hydroxyalkanoate) copolymer containing primarily 3-hydroxyvalerate from simple carbohydrate substrates by Rhodococcus sp. NCIMB 40126. Int. J. Biol. Macromol. 1991, 13, 83–88. [Google Scholar] [CrossRef]
- Tajima, K.; Igari, T.; Nishimura, D.; Nakamura, M.; Satoh, Y.; Munekata, M. Isolation and characterization of Bacillus sp. INT005 accumulating polyhydroxyalkanoate (PHA) from gas field soil. J. Biosci. Bioeng. 2003, 95, 77–81. [Google Scholar] [CrossRef]
- Reddy, S.V.; Thirumala, M.; Mahmood, S.K. Production of PHB and P (3HB-co-3HV) biopolymers by Bacillus megaterium strain OU303A isolated from municipal sewage sludge. World J. Microbiol. Biotechnol. 2008, 25, 391–397. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Increased production of polyhydroxyalkanoates with controllable composition and consistent material properties by fed-batch fermentation. Biochem. Eng. J. 2019, 141, 35–42. [Google Scholar] [CrossRef]
- Martinez, G.A.; Bertin, L.; Scoma, A.; Rebecchi, S.; Braunegg, G.; Fava, F. Production of polyhydroxyalkanoates from dephenolised and fermented olive mill wastewaters by employing a pure culture of Cupriavidus necator. Biochem. Eng. J. 2015, 97, 92–100. [Google Scholar] [CrossRef]
- Choonut, A.; Paichid, N.; Yunu, T.; Sangkharak, K. The production of polyhydroxybutyrate from liquid stillage and its application. In Proceedings of the 2016 World Congress on Sustainable Technologies (WCST), London, UK, 12–14 December 2016; IEEE: New York, NY, USA, 2016; pp. 36–39. [Google Scholar]
- Mumtaz, T.; Yahaya, N.A.; Abd-Aziz, S.; Rahman, N.A.; Yee, P.L.; Shirai, Y.; Hassan, M.A. Turning waste to wealth-biodegradable plastics polyhydroxyalkanoates from palm oil mill effluent—A Malaysian perspective. J. Clean Prod. 2010, 18, 1393–1402. [Google Scholar] [CrossRef]
- Zakaria, M.A.; Tabatabaei, M.; Ghazali, F.M.; Abd-Aziz, S.; Shirai, Y.; Hassan, M.A. Polyhydroxyalkanoate production from anaerobically treated palm oil mill effluent by new bacterial strain Comamonas sp. EB172. World J. Microbiol. Biotechnol. 2010, 26, 767–774. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Ariffin, H.; Johar, N.A.M.; Abd-Aziz, S.; Nishida, H.; Shirai, Y.; Hassan, M.A. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer from wild-type Comamonas sp. EB172. Polym. Degrad. Stab. 2010, 95, 1382–1386. [Google Scholar] [CrossRef]
- Ciesielski, S.; Przybylek, G. Volatilefatty acids influence on the structure of microbial communities producing PHAs. Braz. J. Microbiol. 2014, 45, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Boonsawang, P.; Thongchai, W. Nutrientoptimization of polyhydroxyalkanoate production from palm oil fiber by Ralstonia eutropha TISTR 1095 using response surface methodology. Songklanakarin J. Sci. Technol. 2010, 32, 9–16. [Google Scholar]
- Tsuge, T.; Tanaka, K.; Ishizaki, A. Developmentof a novel method for feeding a mixture of l-lactic acid and acetic acid in fed-batch culture of Ralstonia eutropha for poly-d-3- hydroxybutyrate production. J. Biosci. Bioeng. 2001, 91, 545–550. [Google Scholar] [CrossRef]
- Omar, F.; Aini, N.; Rahman, A.; Hafid, H.; Mumtaz, T.; Yee, P.; Hassan, M. Utilizationof kitchen waste for the production of green thermoplastic polyhydroxybutyrate (PHB) by Cupriavidus necator CCGUG 52238. Afr. J. Microbiol. Res. 2011, 5, 808–1996. [Google Scholar]
- Banu, J.; Rajesh, G.; Ginni, S.; Kavitha, R.; Yukesh Kannah, S.; Adish Kumar, S.K.; Bhatia, G. Kumar Integrated biorefinery routes of biohydrogen: Possible utilization of acidogenic fermentative effluent. Bioresour. Technol. 2021, 319, 124241. [Google Scholar] [CrossRef]
- Szacherska, K.; Oleskowicz-Popiel, P.; Ciesielski, S.; Mozejko-Ciesielska, J. Volatile Fatty Acids as Carbon Sources for Polyhydroxyalkanoates Production. Polymers 2021, 13, 321. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Chen, X.-Y.; Wu, F.-Q.; Chen, J.-C. Polyhydroxyalkanoates(PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Aldor, I.; Keasling, J.D. Metabolic engineering of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composition in recombinant Salmonella enterica Serovar Typhimurium. Biotechnol. Bioeng. 2001, 76, 108–114. [Google Scholar] [CrossRef]
- Al Battashi, H.; Al-Kindi, S.; Gupta, V.K.; Sivakumar, N. Polyhydroxyalkanoate (PHA) Production Using Volatile Fatty Acids Derived from the Anaerobic Digestion of Waste Paper. J. Polym. Environ. 2021, 29, 250–259. [Google Scholar] [CrossRef]
- Amulya, K.; Jukuri, S.; Mohan, S.V. Sustainablemultistage process for enhanced productivity of bioplastics from waste remediation through aerobic dynamic feeding strategy: Process integration for up-scaling. Bioresour. Technol. 2015, 188, 231–239. [Google Scholar] [CrossRef]
- Mohan, M. Polyhydroxyalkanoates Production by Newly Isolated Bacteria Serratia ureilytica Using Volatile Fatty Acids as Substrate: Bio-Electro Kinetic Analysis. J. Microb. Biochem. Technol. 2015, 7, 26–32. [Google Scholar]
- Girdhar, A.; Bhatia, M.; Nagpal, S.; Kanampalliwar, A.; Tiwari, A. ProcessParameters for Influencing Polyhydroxyalkanoate Producing Bacterial Factories: An Overview. J. Pet. Environ. Biotechnol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.W.; Don, T.-M.; Yen, H.-F. Enzymaticextruded starch as a carbon source for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Haloferax mediterranei. Process. Biochem. 2006, 41, 2289–2296. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Rao, Z.-M.; Xue, Y.-F.; Gong, P.; Ji, Y.-Z.; Ma, Y.-H. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Haloarchaeon Halogranumamylolyticum. Appl. Microbiol. Biotechnol. 2015, 99, 7639–7649. [Google Scholar] [CrossRef]
- Chen, H.; Meng, H.; Nie, Z.; Zhang, M. Polyhydroxyalkanoate production from fermented volatile fatty acids: Effect of pH and feeding regimes. Bioresour. Technol. 2013, 128, 533–538. [Google Scholar] [CrossRef]
- Aditi, S.; Souza Shalet, N.M.D.; Pranesh, R.; Katyayini, T. Microbialproduction of polyhydroxyalkanoates (PHA) from novel sources: A review. Int. J. Biol. Macromol. 2015, 4, 16–28. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A. Continuousproduction mode as a viable process-engineering tool for efficient poly (hydroxyalkanoate)(PHA) bio-production. Chem. Biochem. Eng. Q. 2014, 28, 65–77. [Google Scholar]
- Hafuka, A.; Sakaida, K.; Satoh, H.; Takahashi, M.; Watanabe, Y.; Okabe, S. Effectof feeding regimens on polyhydroxybutyrate production from food wastes by Cupriavidus necator. Bioresour. Technol. 2011, 102, 3551–3553. [Google Scholar] [CrossRef] [Green Version]
- Morgan-Sagastume, F.; Valentino, F.; Hjort, M.; Cirne, D.; Karabegovic, L.; Gerardin, F.; Johansson, P.; Karlsson, A.; Magnusson, P.; Alexandersson, T.; et al. Polyhydroxyalkanoate (PHA) production from sludge and municipal wastewater treatment. Water Sci. Technol. 2014, 69, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Valentino, F.; Karabegovic, L.; Majone, M.; Morgan-Sagastume, F.; Werker, A. Polyhydroxyalkanoate(PHA) storage within a mixed-culture biomass with simultaneous growth as a function of accumulation substrate nitrogen and phosphorus levels. Water Res. 2015, 77, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, S.; Serrano, A.; Pantión, A.A.; Alonso-Fariñas, B. Challengesof scaling-up PHA production from waste streams. A review. J. Environ. Manag. 2018, 205, 215–230. [Google Scholar] [CrossRef] [Green Version]
- Sirohi, R.; Pandey, J.; Gaur, V.K.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef]
- Beun, J.; Dircks, K.; Van Loosdrecht, M.; Heijnen, J. Poly-β-hydroxybutyrate metabolism in dynamically fed mixed microbial cultures. Water Res. 2002, 36, 1167–1180. [Google Scholar] [CrossRef]
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.; Reis, M.A. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160. [Google Scholar] [CrossRef]
- Johnson, K.; Jiang, Y.; Kleerebezem, R.; Muyzer, G.; Van Loosdrecht, M.C.M. Enrichment of a Mixed Bacterial Culture with a High Polyhydroxyalkanoate Storage Capacity. Biomacromolecules 2009, 10, 670–676. [Google Scholar] [CrossRef]
- Chua, A.S.M.; Takabatake, H.; Satoh, H.; Mino, T. Production of polyhydroxyalkanoates (PHA) by activated sludge treating municipal wastewater: Effect of pH, sludge retention time (SRT), and acetate concentration in influent. Water Res. 2003, 37, 3602–3611. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Christensson, M.; Welander, T. Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour. Technol. 2008, 99, 509–516. [Google Scholar] [CrossRef]
- Farghaly, A.; Enitan, A.M.; Kumari, S.; Bux, F.; Tawfik, A. Polyhydroxyalkanoates production from fermented paperboard mill wastewater using acetate-enriched bacteria. Clean Technol. Environ. Policy 2016, 19, 935–947. [Google Scholar] [CrossRef]
- Liao, Q.; Gao, M.; Ran, Y.; Gao, M.; She, Z.; Zhao, Y.; Liu, Y. Optimization of polyhydroxyalkanoates (PHA) synthesis with heat pretreated waste sludge. Waste Manag. 2018, 82, 15–25. [Google Scholar] [CrossRef]
- Burniol-Figols, A.; Varrone, C.; Le, S.B.; Daugaard, A.E.; Skiadas, I.V.; Gavala, H.N. Combined polyhydroxyalkanoates (PHA) and 1,3-propanediol production from crude glycerol: Selective conversion of volatile fatty acids into PHA by mixed microbial consortia. Water Res. 2018, 136, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Yin, F.; Ma, X. Towards biodegradable polyhydroxyalkanoate production from wood waste: Using volatile fatty acids as conversion medium. Bioresour. Technol. 2020, 299, 122629. [Google Scholar] [CrossRef]
- Fradinho, J.; Oehmen, A.; Reis, M. Improving polyhydroxyalkanoates production in phototrophic mixed cultures by optimizing accumulator reactor operating conditions. Int. J. Biol. Macromol. 2019, 126, 1085–1092. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.V.; Mohan, S.V. Effectof substrate load and nutrients concentration on the polyhydroxyalkanoates (PHA) production using mixed consortia through wastewater treatment. Bioresour. Technol. 2012, 114, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Krishnan, S.; Sagarika, V.K.; Tharayil, A.; Kalarikkal, N.; Thomas, S. Bionanocomposites as industrial materials, current and future perspectives: A review. Emergent Mater. 2020, 3, 711–725. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, P.; Ray, S.; Kalia, V.C. Challenges and opportunities for customizing polyhydroxyalkanoates. Indian J. Microbiol. 2015, 55, 235–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalheiro, J.M.B.T.; Raposo, R.S.; de Almeida, M.C.M.D.; Teresa Cesário, M.; Sevrin, C.; Grandfils, C.; da Fonseca, M.M.R. Effect of cultivation parameters on the production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour. Technol. 2012, 111, 391–397. [Google Scholar] [CrossRef]
- Tran, T.T.; Charles, T.C. Lacticacid containing polymers produced in engineered Sinorhizobium meliloti and Pseudomonas putida. PLoS ONE 2020, 15, e0218302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Li, W.; Zhang, Z.-Z.; Tan, T.-W.; Li, Z.-J. Metabolicengineering of Escherichia coli for the synthesis of polyhydroxyalkanoates using acetate as a main carbon source. Microb. Cell Fact. 2018, 17, 102. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Lee, S.Y. Production of poly(3-hydroxybutyrate) by fed-batch culture of filamentation-suppressed recombinant Escherichia coli. Appl. Environ. Microbiol. 1997, 63, 4765–4769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldor, I.S.; Kim, S.-W.; Prather, K.L.J.; Keasling, J.D. Metabolic Engineering of a Novel Propionate-Independent Pathway for the Production of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) in Recombinant Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2002, 68, 3848–3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.F.; Gomez, J.G.C.; Oliveira, M.S.; Torres, B.B. Propionicacid metabolism and poly-3-hydroxybutyrate-co-3-hydroxyvalerate (P3HB-co-3HV) production by Burkholderia sp. J. Biotechnol. 2000, 76, 165–174. [Google Scholar] [CrossRef]
- Liu, X.W.; Wang, H.H.; Chen, J.Y.; Li, X.T.; Chen, G.Q. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by recombinant Escherichia coli harboring propionyl-CoA synthase gene (prpE) or propionate permease gene (prpP). Biochem. Eng. J. 2009, 43, 72–77. [Google Scholar] [CrossRef]






| Volatile Fatty Acid | Chemical Structure and Formula | Chemical Properties | Production Methods | Global Market Size and CAGR | Usage/Application |
|---|---|---|---|---|---|
| Acetic acid |  CH3COOH | MW: 60.05 Density:1.05 pKa: 4.76 | Methanol carbonylation, oxidation of acetaldehyde and ethylene, oxidative and anaerobic fermentation | USD 9.3 billion in 2020; CAGR of 5.2% | Vinyl acetate monomer as adhesives, dyes, food additives, vinegar, ester manufacture |
| Butyric acid |  CH3 (CH2)2COOH | MW: 88.11 Density:0.96 pKa: 4.82 | Oxidation of butyraldehyde, extraction from butter, anaerobic fermentation | USD 175 million in 2020; CAGR of 13.2% | Food additives (animal, human), chemical precursors, solvents, flavouring agents |
| Propionic acid |  CH3CH2COOH CH3CH2COOH | MW:74.08 Density:0.99 pKa: 4.88 | Hydrocarboxylation of ethylene, aerobic oxidation of propionaldehyde, direct oxidation of hydrocarbons, anaerobic fermentation | USD 1.53 billion in 2020; CAGR of 2.7% | Food additives, flavoring, pharmaceuticals, animal feed supplements, fishing bait additives |
| Lactic acid |  CH3CHOHCOOH CH3CHOHCOOH | MW:90.08 Density:1.20 pKa:3.86 | Chemical synthesis, anaerobic fermentation | USD 2.7 billion in 2020, CAGR of 8.0% | Polymers (polylactic acid) production, food products, additives, cleaning products |
| Valeric acid |  CH3(CH2)3COOH CH3(CH2)3COOH | MW:102.13 Density:0.93 pKa: 4.84 | Oxo process from 1-butene and syngas, anaerobic fermentation | USD 15.06 billion in 2020; CAGR of 5.3% | Food additives, in perfumes, cosmetics, and foodstuffs |
| Company | Carbon Source | Type of PHA | Final PHA (% CDW) | Production (Tones/Annum) | Year | Potential Uses |
|---|---|---|---|---|---|---|
| Tianjin Northern Food, Tianjin, China | Glucose | PHB | >80% | Pilot scale | 1990s | Raw materials |
| Chemie Linz, btf, Linz, Austria Biomers, Ulm, Germany | Glucose or sucrose | PHB | >75% | 20–100 | 1980s | Packaging and drug delivery |
| Jiangsu LanTian, Taizhou, China | Glucose | PHB | >80% | 20–100 | 1990s | Packaging and drug delivery |
| ICI, UK Zhejiang Tianan, Hangzhou, China | Glucose + propionate | PHBV | >75% | 300– 2000 | 1980s to 1990s 1990s to present | Packaging and raw materials |
| Metabolix, Woburn, MA, USA | Glucose + | P3HB4HB | >75% | Unknown | 1980s to present | Packaging |
| P&G, Kaneka, Osaka, Japan | Fatty acids | PHBHHx | >80% | Unknown | 1990s to present | Packaging |
| P&G, Jiangmen Biotech Ctr, Jiangmen, China | Lauric acid | PHBHHx | <50% | Unknown | 1990s | Raw materials |
| Shandong Lukang, Jining, China | Lauric acid | PHBHHx | >50% | Pilot scale | 2005 to present | Raw materials and packaging |
| ETH, Zürich, Switzerland | Fatty acids | MCL PHA | >60% | NA | Raw materials and packaging | |
| Biocycles, São Paulo, Brazil | Sucrose | PHB | >50% | 100 | 1990s to present | Raw materials |
| Employed Substrate | Microorganism | Fermentation Type | DCW (g/L) | PHA Accumulation (%) | PHA Yield (g/L) | Type of PHA | Reference |
|---|---|---|---|---|---|---|---|
| Acetic acid | Ralstonia eutropha ATCC 17699 | Shaking flask | 5.4 | 30.8 | 1.66 | P(3HB) | [37] |
| Propionic acid | Ralstonia eutropha ATCC 17699 | Shaking flask | 14.0 | 29.3 | 4.10 | P(3HB) | [37] |
| Butyric acid | Ralstonia eutropha ATCC 17699 | Shaking flask | 14.5 | 31.9 | 4.62 | P(3HB) | [37] |
| Propionic acid + glucose Propionic acid + glucose Acetic acid + propionic acid + glucose | Pseudomonas sp. ST2 Bacillus sp. CS8 Pseudomonas sp. ST2 + Bacillus sp. CS8 | Shaking flask | ND | 34.0 24.0 35.0 | ND | P(3HB-co-3HV) | [38] |
| Acetic acid Propionic acid Butyric acid Valeric acid | Methylocystis hirsuta DSM 18500 | Shaking flask | ND | 2.4 1.1 1.8 9.0 | ND | P(3HB-co-3HV) | [39] |
| Acetic acid + biogas Propionic acid + biogas Butyric acid + biogas Valeric acid + biogas | Methylocystis hirsuta DSM 18500 | Shaking flask | ND | 52.3 47.9 52.2 53.8 | ND | P(3HB) P(3HB-co-3HV) P(3HB) P(3HB-co-3HV) | [39] |
| Acetic acid | Ralstonia eutropha H16 (DSM 428) | Shaking flask | ND | 33.3 | 0.5 | P(3HB) | [46] |
| Acetic acid | Clostridiumautoethanogenum | Fed-batch | ND | 24 | ND | P(3HB) | [47] |
| Acetic acid + propionic acid + butyric acid | Ralstonia eutropha ATCC 17699 | Batch | 1.2 | 25.0 | 0.30 | P(3HB-co-3HV) | [41] |
| Acetic acid + propionic acid + butyric acid | Ralstonia eutropha KCTC 2658 | Batch | 1.5 | 50 | 0.75 | P(3HB-co-3HV) | [41] |
| Acetic acid Acetic acid Acetic acid Valeric acid | Corynebacterium hydrocarboxydans Nocardia lucida Rhodococcus sp. NCIMB 40126 | Batch | ND | 21.0 20.0 29.0 43.0 | ND | P(3HB-co-3HV) P(3HB-co-3HV) P(3HB-co-3HV) P(3HB-co-3HV) | [48] |
| Butyric acid Valeric acid | Bacillus sp. INT005 | Shaking flask | 0.8 0.7 | 31.5 18.8 | 0.25 0.13 | P(3HB) P(3HB-co-3HV) | [49] |
| Propionic acid + glucose Propionic acid + glycerol | Bacillus megaterium OU303A | Shaking flask | ND | 62.4 57.2 | ND | P(3HB) | [50] |
| Butyric acid + valeric acid + Tween 20 | Haloferax mediterranei | Fed batch | ND | 58.9 | ND | P(3HB-co-3HV) | [51] |
| Olive mill wastewater effluent rich in acetic, propionic, and butyric acid | Cupriavidus necator | Two-stage batch cultivation | 2.0 | 55.0 | 1.1 | P(3HB-co-3HV) | [52] |
| Acetic acid 0.5 g/L 5.0 g/L | Bacillus cereus strain HY | Shaking flask | 1.82 2.94 | 40.2 49.8 | 0.73 1.46 | P(3HB) | [35] |
| Acetic, propionic, butyric | Alcaligenes eutrophus | Shaking flask | 6.64 | 86.5 | 5.75 | P(3HB) | [53] |
| Acetic, propionic and n-butyric acids. | Comamonas sp. EB172 | Fed-batch | 7.2 | 90 | 6.48 | P(3HB-co-3HV) | [54] |
| Acetic, propionic and n-butyric acids. | Comamonas sp. EB172 | Fed-batch | 9.8 | 59 | 5.78 | P(3HB-co-3HV) | [55] |
| Acetic, propionic and n-butyric acids. | Comamonas sp. EB172 | Shake flask | 3 | 20 | 0.6 | P(3HB-co-3HV) | [56] |
| Acetic, propionic, butyric | Thauera sp. Paracoccus denitrificans | Sequencing batch reactor | ND | 34.2 | 227.8 mg/L 673 mg/L | P(3HB) P(3HB-co-3HV) | [57] |
| Propionic and butyric acid | Ralstonia eutropha | Batch | 1.53 | 46.5 | 0.7 | P(3HB) | [58] |
| Lactic acid and acetic acid | Ralstonia eutropha | Fed batch | 75 | 73.1 | 54.8 | P(3HB) | [59] |
| Lactic acid and acetic acid | Cupriavidus necator CGUG 52238 | Batch | ND | 84.54 (w/w) | 0.79 g/g | P(3HB) | [60] |
| Employed Substrate | Inoculum Source | Fermentation Type | PHA Accumulation (%) | PHA Yield (g/L) | Type of PHA | Reference |
|---|---|---|---|---|---|---|
| Fermented molasses | Mixed activated sludge culture | Sequencing batch reactor | 66.0 | ND | P(3HB-co-3HV) | [3] |
| Acidogenic effluent | Enriched mixed cultures | Batch | 54 | ND | P(3HB-co-3HV) | [31] |
| Fermented food waste + dewatered sludge | Mixed activated sludge culture | Batch | 64.5 | ND | P(3HB-co-3HV) | [71] |
| Acetic acid | Mixed activated sludge culture | Sequencing batch reactor | 40.0 | ND | P(3HB) | [79] |
| Acetic acid | Mixed activated sludge culture | Batch reactor | 78.5 | 5–180 Cmmol/l for acetate | P(3HB) | [80] |
| Acetic acid | Mixed microbial culture | Acetate-fed sequencing batch reactor | 89.0 | ND | P(3HB) | [81] |
| Municipal wastewater + acetic acid | Mixed activated sludge culture | Sequencing batch reactors | 30.0 | 28 mg C/g SS/h | P(3HB) | [82] |
| Fermented paper mill wastewater | Mixed activated sludge culture | Batch | 48.0 | 0.11 kg of PHA/kg of COD (treated influent) | P(3HB-co-3HV) | [83] |
| Fermented paperboard mill wastewater | Mixed microbial culture | Sequencing batch reactors | 67.4 | 0.46 ± 0.09 C-mol C-mol−1 | P(3HB-co-3HV) | [84] |
| Sludge hydrolysis liquid | Heat pretreated waste sludge | Sequencing batch reactor | 24.1 | 0.46 mg COD/mg COD | P(3HB-co-3HV) | [85] |
| Fermented crude glycerol | Mixture of equivalent ratio of anaerobic sludge and aerobic sludge | Sequencing batch reactor | 76.0 | 0.84 g COD PHA/g COD S | P(3HB-co-3HV) | [86] |
| Fermented wood waste | Acidogenic sludge | Batch | 50.3 | 0.71 g COD PHA/g COD VFAs | P(3HB-co-3HV) | [87] |
| Fermented cheese whey | Phototrophic mixed cultures | Sequencing batch reactors | 30.0 | 0.83 ± 0.07 Cmol-PHB/Cmol-Acet | P(3HB-co-3HV) | [88] |
| Fermented Food waste | Acidogenic mixed bacteria | Batch | 23.7 | 0.168 g PHACOD/g WWCOD | P(3HB-co-3HV) | [66] |
| Fermented Food waste | Industrial wastewater | Fed-batch | 39.6 | ND | P(3HB-co-3HV) | [89] |
| Employed Substrate | Microorganism | Fermentation Type | PHA Yield (g/L) | Type of PHA | Reference |
|---|---|---|---|---|---|
| Propionic acid | Herbaspirillum seropedicae Z69 2-methylcitrate synthase (PrpC) gene | Shaking flask | 3HV yield 0.80 g/g | PHBV | [19] |
| Acetic acid | Bacillus cereus strain HY-3 | Shaking flask | PHB content—49.2% | PHB | [35] |
| Glycerol propionic acid | Salmonella enterica serovar Typhimurium | Shake flask | 3HV yield 19.4 (mol%) | P(3HB-co-3HV) | [64] |
| Acetate | Escherichia coli Overexpressed pta-ackA and acs genes | Shaking flask | 1.27 g/L | P3HB | [94] |
| Glycerol | E. XL1-Blue filamentation-suppressed FtsZ | Fed-batch | 149 g/L | PHB | [95] |
| Glycerol + propionic acid | S. enterica propionyl-CoA prpC, as a host | Shaking flask | 34.2 ± 15 (%DCW) | PHBV | [96] |
| Propionic acid | Burkholderia sp. IPT 101 propionyl- prp mutants | Batch | Y3HV:Prop 1.20 g g−1 | PHBV | [97] |
| Glucose propionate | Recombinant E. coli XL10-Gold/pBHR68 | Fed-batch | 3HV yield 0.31 g/g | P(3HB-co-3HV) | [98] |
| Glucose propionate | Recombinant E. coli XL10-Gold/pBHR68-prpP | Fed-batch | 3HV yield 0.46 g/g | P(3HB-co-3HV) | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kumar, M.; Bharagava, R.N.; Varjani, S.; Kadam, A.A.; Ghodake, G.S.; Palem, R.R.; Mulla, S.I.; et al. An Overview of Recent Advancements in Microbial Polyhydroxyalkanoates (PHA) Production from Dark Fermentation Acidogenic Effluents: A Path to an Integrated Bio-Refinery. Polymers 2021, 13, 4297. https://doi.org/10.3390/polym13244297
Saratale RG, Cho S-K, Saratale GD, Kumar M, Bharagava RN, Varjani S, Kadam AA, Ghodake GS, Palem RR, Mulla SI, et al. An Overview of Recent Advancements in Microbial Polyhydroxyalkanoates (PHA) Production from Dark Fermentation Acidogenic Effluents: A Path to an Integrated Bio-Refinery. Polymers. 2021; 13(24):4297. https://doi.org/10.3390/polym13244297
Chicago/Turabian StyleSaratale, Rijuta Ganesh, Si-Kyung Cho, Ganesh Dattatraya Saratale, Manu Kumar, Ram Naresh Bharagava, Sunita Varjani, Avinash A. Kadam, Gajanan S. Ghodake, Ramasubba Reddy Palem, Sikandar I. Mulla, and et al. 2021. "An Overview of Recent Advancements in Microbial Polyhydroxyalkanoates (PHA) Production from Dark Fermentation Acidogenic Effluents: A Path to an Integrated Bio-Refinery" Polymers 13, no. 24: 4297. https://doi.org/10.3390/polym13244297