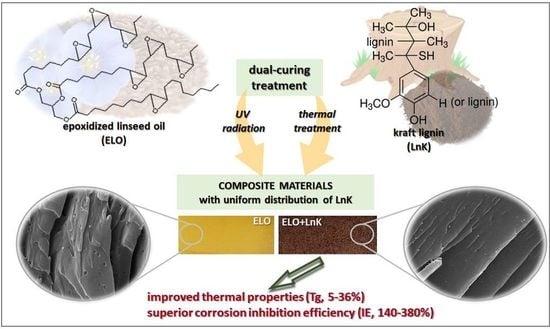
Composite Materials from Renewable Resources as Sustainable Corrosion Protection Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of ELO
2.2.2. Preparation of the ELO_LnK Composite Coatings
2.3. Characterization
2.3.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.3.2. Gel Fraction Measurements (GF)
2.3.3. Fourier Transform Infrared Spectrometry (FTIR)
2.3.4. Thermo-Gravimetric Analysis (TGA)
2.3.5. Dynamic Mechanical Analysis (DMA)
2.3.6. Contact Angle Measurements (CA) and Water Absorption Degree (WAD)
2.3.7. Scanning Electron Microscopy (SEM)
2.3.8. Electrochemical Measurements
3. Results and Discussion
3.1. ELO Characterization
3.2. ELO-LnK Composite Synthesis and Characterization
3.3. FTIR Spectrometry
3.4. Thermogravimetric Analysis
3.5. Dynamic-Mechanical Analysis
3.6. Morphology Investigation
3.7. Water Affinity
3.8. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, P.; Ma, Y.; Kong, Q.; Xu, L.; Li, Q.; Zhou, Y. Progress in development of epoxy resin systems based on biomass resources. Green Mater. 2020, 8, 6–23. [Google Scholar] [CrossRef]
- Ramon, E.; Sguazzo, C.; Moreira, M.G.P.P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Conti Silva, J.A.; Moreira Grilo, L.; Gandini, A.; Martins Lacerda, T. The Prospering of Macromolecular Materials Based on Plant Oils within the Blooming Field of Polymers from Renewable Resources. Polymers 2021, 13, 1722. [Google Scholar] [CrossRef]
- Motta Neves, R.; Ornaghi, H.L., Jr.; José Zattera, A.; Campos Amico, S. Recent studies on modified cellulose/nanocellulose epoxy composites: A systematic review. Carbohydr. Polym. 2021, 255, 117366. [Google Scholar] [CrossRef] [PubMed]
- Matykiewicz, D. Hybrid Epoxy Composites with Both Powder and Fiber Filler: A Review of Mechanical and Thermomechanical Properties. Materials 2020, 13, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally friendly anticorrosive polymeric coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Hsissou, R. Review on epoxy polymers and its composites as a potential anticorrosive coating for carbon steel in 3.5% NaCl solution: Computational approaches. J. Mol. Liq. 2021, 336, 116307. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; Gouri, M.E.; Sherif, M.E.; Ebenso, E.E. Epoxy resins as anticorrosive polymeric materials: A review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Noè, C.; Iannucci, L.; Malburet, S.; Graillot, A.; Sangermano, M.; Grassini, S. New UV-Curable Anticorrosion Coatings from Vegetable Oils. Macromol. Mater. Eng. 2021, 306, 2100029. [Google Scholar] [CrossRef]
- Noè, C.; Hakkarainen, M.; Sangermano, M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymers 2021, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, S.; Dong, X.; Liu, K.; Zhao, X.; Kong, F. Construction of a Novel Lignin-Based Quaternary Ammonium Material with Excellent Corrosion Resistant Behavior and Its Application for Corrosion Protection. Materials 2019, 12, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciales, A.; Haile, T.; Ahvazi, B.; Ngo, T.D.; Wolodko, J. Performance of green corrosion inhibitors from biomass in acidic media. Corros. Rev. 2018, 36, 239–266. [Google Scholar] [CrossRef]
- Kulkarni, P.; Ponnappa, C.B.; Doshi, P.; Rao, P.; Balaji, S. Lignin from termite frass: A sustainable source for anticorrosive applications. J. Appl. Electrochem. 2021, 51, 1491–1500. [Google Scholar] [CrossRef]
- Ott, M.W.; Dietz, C.; Trosien, S.; Mehlhase, S.; Bitsch, M.J.; Nau, M.; Meckel, T.; Geissler, A.; Siegert, G.; Huong, J.; et al. Co-curing of epoxy resins with aminated lignins: Insights into the role of lignin homo crosslinking during lignin amination on the elastic properties. Holzforschung 2021, 75, 390–398. [Google Scholar] [CrossRef]
- Balanuca, B.; Stan, R.; Hanganu, A.; Iovu, H. Novel Linseed Oil-Based Monomers: Synthesis and Characterization. UPB Sci. Bull. B Chem. Mater. Sci. 2014, 76, 129–140. [Google Scholar]
- Balanuca, B.; Lungu, A.; Hanganu, A.; Stan, L.R.; Vasile, E.; Iovu, H. Hybrid nanocomposites based on POSS and networks of methacrylated camelina oil and various PEG derivatives. Eur. J. Lipid Sci. Technol. 2014, 116, 458–469. [Google Scholar] [CrossRef]
- Balanuca, B.; Stan, R.; Hanganu, A.; Lungu, A.; Iovu, H. Design of new camelina oil-based hydrophilic monomers for novel polymeric materials. J. Am. Oil Chem. Soc. 2015, 92, 881–891. [Google Scholar] [CrossRef]
- Balanuca, B.; Stan, R.; Lungu, A.; Vasile, E.; Iovu, H. Hybrid networks based on epoxidized camelina oil. Des. Monomers Polym. 2017, 20, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Balanuca, B.; Ghebaur, A.; Stan, R.; Vuluga, D.M.; Vasile, E.; Iovu, H. New hybrid materials based on double-functionalized linseed oil and halloysite. Polym. Adv. Technol. 2018, 29, 1744–1752. [Google Scholar] [CrossRef]
- Healey, P.; Sadleir, R.M.F.S. The construction of rectal electrodes. J. Reprod. Fertil. 1966, 11, 299–301. [Google Scholar] [CrossRef] [Green Version]
- Barker, I.A.; Dove, A.P. Triarylsulfonium hexafluorophosphate salts as photoactivated acidic catalyst for ring-opening polymerization. Chem. Commun. 2013, 49, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Medina, F.J.; Pizzi, A.; Basso, M.C.; Delmotte, L.; Abdalla, S. Polycondensation Resins by Lignin Reaction with (Poly)amines. J. Renew. Mater. 2017, 5, 388–399. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; Snijder, M.H.B.; Kranenbarg, A.; Keijsers, E.R.P.; de Jong, E.; Stigsson, L.L. Characterisation and application of NovaFiber lignin. Ind. Crops Prod. 2004, 20, 191–203. [Google Scholar] [CrossRef]
- Rozman, H.D.; Koay, E.L.; Tay, G.S. Preliminary Study on the Utilization of Lignin as Filler in Ultra-Violet (UV) Curable System. J. Appl. Polym. Sci. 2011, 120, 2527–2533. [Google Scholar] [CrossRef]
- Available online: https://polymerdatabase.com/polymer%20classes/Lignin%20type.html (accessed on 26 September 2021).
- Patil, C.K.; Rajput, S.D.; Marathe, R.J.; Kulkarni, R.D.; Phadnis, H.; Sohn, D.; Mahulikar, P.P.; Gite, V.V. Synthesis of bio-based polyurethane coatings from vegetable oil and dicarboxylic acids. Prog. Org. Coat. 2017, 106, 87–95. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Dastpak, A.; Yliniemi, K.; de Oliveira Monteiro, M.C.; Höhn, S.; Virtanen, S.; Lundström, M.; Wilson, B.P. From Waste to Valuable Resource: Lignin as a Sustainable Anti-Corrosion Coating. Coatings 2018, 8, 454. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Hu, Z.; Shi, J.; Chen, G.; Zhang, Q.; Weng, Z.; Wu, K.; Lu, M. Green synthesis of graphene with the assistance of modified lignin and its application in anticorrosive waterborne epoxy coatings. Appl. Surf. Sci. 2019, 484, 759–770. [Google Scholar] [CrossRef]









| N2 Atmosphere | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Code | Weight Loss | Degradation Steps | Weight Change at 700 °C (%) | ||||||||
| Td a | Tmax b | ||||||||||
| 3% (°C) | 10% (°C) | 30% (°C) | 50% (°C) | 1 (°C) | 2 (°C) | 3 (°C) | |||||
| Sr | 185 | 223 | 320 | 358 | 234 | 365 | 448 s | 99 | |||
| S1 | 197 | 247 | 339 | 366 | 243 | 365 | 440 s | 96 | |||
| S2 | 192 | 242 | 336 | 364 | 230 | 362 | 448 s | 91 | |||
| S3 | 197 | 256 | 343 | 370 | 250 | 363 | 449 s | 88 | |||
| LnK | 159 | 258 | 355 | 502 | - | 353 | - | 61 | |||
| Air Atmosphere | |||||||||||
| Sample Code | Weight Loss | Degradation Steps | Weight Change at 700 °C (%) | ||||||||
| Td a | Tmax b | ||||||||||
| 3% (°C) | 10% (°C) | 30% (°C) | 50% (°C) | 1 (°C) | 2 (°C) | 3 (°C) | 4 (°C) | ||||
| Sr | 193 | 242 | 336 | 383 | 230 | 333 | 375 421 s | 527 | 88 | ||
| S1 | 199 | 256 | 346 | 387 | 260 | 339 | 378 426 s | 520 | 85 | ||
| S2 | 191 | 243 | 332 | 374 | 243 | 338 | 376 417 s | 509 | 98 | ||
| S3 | 182 | 229 | 325 | 373 | 224 | 336 | 376 395 429 s | 503 | 98 | ||
| LnK | 86 | 255 | 395 | 436 | - | - | 448 | - | 93 | ||
| Sample | Tg c (°C) | GF d (%) | Θ e (°) |
|---|---|---|---|
| Sr | 55 | 92.06 ± 0.54 | 84.82 ± 1.72 |
| S1 | 58 | 93.13 ± 0.24 | 77.89 ± 4.07 |
| S2 | 62 | 93.95 ± 0.22 | 73.97 ± 1.46 |
| S3 | 75 | 94.99 ± 0.30 | 71.38 ± 1.95 |
| Sample Code | Ecorr (mV/SSCS) | icorr (µA/cm2) | Rp (kohm * cm2) | βa (mV) | −βc (mV) | Correlation Coefficient | IE (%) |
|---|---|---|---|---|---|---|---|
| OL * | −646 | 1.5531 | 12.64 | 75.3 | 257.3 | 0.9999 | - |
| Sr | −584 | 0.5562 | 52.71 | 180.8 | 185.0 | 0.9977 | 64.19 |
| S1 | −581 | 0.0015 | 19,930.00 | 186.1 | 188.3 | 0.9974 | 99.90 |
| S2 | −558 | 0.1036 | 282.20 | 185.1 | 183.3 | 0.9977 | 93.33 |
| S3 | −523 | 0.0601 | 450.26 | 171.0 | 170.6 | 0.9977 | 96.13 |
| Sample Code | A * | B * | ||||||
|---|---|---|---|---|---|---|---|---|
| R1 (ohm * cm2) | R2 (kohm * cm2) | Cdl (pF/cm2) | IE (%) | R1 (ohm * cm2) | R2 (kohm * cm2) | Cdl (pF/cm2) | IE (%) | |
| OL | 111.80 | 6.48 | 43.69 106 | - | 45.83 | 37.50 | 84.87 106 | - |
| Sr | 63.83 | 41.43 | 307.30 | 84.35 | 38.52 | 49.53 | 321.20 | 24.29 |
| S1 | 92.99 | 20,600.00 | 691.90 | 99.97 | 41.63 | 17,350.00 | 366.80 | 99.78 |
| S2 | 132.00 | 405.90 | 43.90 | 98.40 | 224.00 | 596.30 | 53.37 | 93.71 |
| S3 | 70.04 | 390.40 | 290.10 | 98.34 | 40.90 | 501.70 | 225.80 | 92.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komartin, R.S.; Balanuca, B.; Necolau, M.I.; Cojocaru, A.; Stan, R. Composite Materials from Renewable Resources as Sustainable Corrosion Protection Coatings. Polymers 2021, 13, 3792. https://doi.org/10.3390/polym13213792
Komartin RS, Balanuca B, Necolau MI, Cojocaru A, Stan R. Composite Materials from Renewable Resources as Sustainable Corrosion Protection Coatings. Polymers. 2021; 13(21):3792. https://doi.org/10.3390/polym13213792
Chicago/Turabian StyleKomartin, Raluca Sanda, Brindusa Balanuca, Madalina Ioana Necolau, Anca Cojocaru, and Raluca Stan. 2021. "Composite Materials from Renewable Resources as Sustainable Corrosion Protection Coatings" Polymers 13, no. 21: 3792. https://doi.org/10.3390/polym13213792