Effects of UV-C and Edible Nano-Coating as a Combined Strategy to Preserve Fresh-Cut Cucumber
Abstract
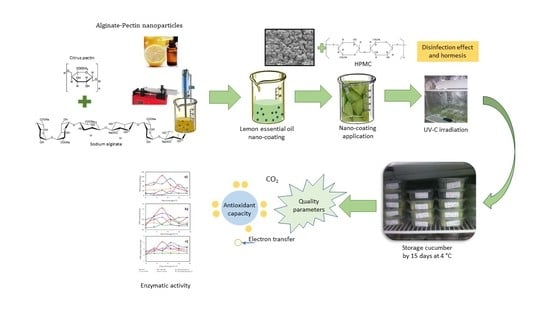
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cucumbers
2.3. Preparation of Lemon Essential Oil Nanoparticles
2.4. Characterization of the LEO-A-P-NP
2.5. Encapsulation Efficiency (EE)
2.6. Preparation of the Coating Dispersion
2.7. UV-C Irradiation Conditions and Application of the Coating
2.8. Application of the Nano-Coating and Packaging of the Cucumber
2.9. Headspace Gas Analysis
2.10. Weight Loss, Total Soluble Solids (TSS), and pH
2.11. Firmness and Color
2.12. Chlorophyll Content
2.13. Ascorbic Acid
2.14. Total Phenol Content (TPC)
2.15. Radical Scavenging Activity (DPPH)
2.16. Enzymatic Activity
2.16.1. Peroxidase Activity (POD)
2.16.2. Pectin Methylesterase (PME)
2.16.3. Polyphenol Oxidase (PPO)
2.17. Microbiological Analyses
2.18. Statistical Analyses
3. Results
3.1. Characterization of the Alginate-Pectin Nanoparticles
3.2. CO2 Concentration in Fresh-Cut Cucumber
3.3. Weight Loss
3.4. Total Soluble Solids and pH
3.5. Chlorophyll
3.6. Color of the Fresh-Cut Cucumber
3.7. Firmness of the Fresh-Cut Cucumber
3.8. Ascorbic Acid Degradation during Storage at 4 °C
3.9. Total Phenol Content
3.10. DPPH Antioxidant Capacity
3.11. Polyphenol Oxidase (PPO) Activity
3.12. Peroxidase Activity (POD)
3.13. Pectin Methylesterase (PME) Activity
3.14. Microbiological Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, X.; Zhang, M.; Zhan, Z.; Adhikari, B. Changes in Quality Characteristics of Fresh-Cut Cucumbers as Affected by Pressurized Argon Treatment. Food Bioprocess Technol. 2014, 7, 693–701. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Chen, H. Effect of Ultrasound Treatment Combined with Carbon Dots Coating on the Microbial and Physicochemical Quality of Fresh-Cut Cucumber. Food Bioprocess Technol. 2020, 13, 648–660. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Lee, W. Influence of Chitosan Coating and Packaging Materials on the Quality Characteristics of Fresh-Cut Cucumber. Korean J. Food Preserv. 2019, 26, 371–380. [Google Scholar] [CrossRef] [Green Version]
- González-Reza, R.M.; García-Betanzos, C.I.; Sánchez-Valdes, L.I.; Quintanar-Guerrero, D.; Cornejo-Villegas, M.A.; Zambrano-Zaragoza, M.L.; González, R.M.; García, C.I.; Sánchez, L.I. The Functionalization of Nanostructures and Their Potential Applications in Edible Coatings. Coatings 2018, 8, 160. [Google Scholar] [CrossRef] [Green Version]
- Zambrano-Zaragoza, M.L.; Gutiérrez-Cortez, E.; Del Real, A.; González-Reza, R.M.; Galindo-Pérez, M.J.; Quintanar-Guerrero, D. Fresh-Cut Red Delicious Apples Coating Using Tocopherol/Mucilage Nanoemulsion: Effect of Coating on Polyphenol Oxidase and Pectin Methylesterase Activities. Food Res. Int. 2014, 62, 974–983. [Google Scholar] [CrossRef]
- Formiga, A.S.; Pinsetta, J.S.; Pereira, E.M.; Cordeiro, I.N.F.; Mattiuz, B.H. Use of Edible Coatings Based on Hydroxypropyl Methylcellulose and Beeswax in the Conservation of Red Guava ‘Pedro Sato’. Food Chem. 2019, 290, 144–151. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. The Efficacy of Edible Film from Konjac Glucomannan and Saffron Petal Extract to Improve Shelf Life of Fresh-Cut Cucumber. Food Sci. Nutr. 2020, 8, 3128–3137. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Lopez, A.D.R.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Gutiérrez-Cortez, E. Effect of Nano-Edible Coating Based on Beeswax Solid Lipid Nanoparticles on Strawberry’s Preservation. Coatings 2020, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Bustos, C.R.O.; Alberti, R.F.V.; Matiacevich, S.B. Edible Antimicrobial Films Based on Microencapsulated Lemongrass Oil. J. Food Sci. Technol. 2016, 53, 832–839. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and Characterization of Grass Carp Collagen-Chitosan-Lemon Essential Oil Composite Films for Application as Food Packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Maringgal, B.; Hashim, N.; Mohamed Amin Tawakkal, I.S.; Muda Mohamed, M.T. Recent Advance in Edible Coating and Its Effect on Fresh/Fresh-Cut Fruits Quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Miranda-Linares, V.; Quintanar-Guerrero, D.; Del Real, A.; Zambrano-Zaragoza, M.L.L. Spray-Drying Method for the Encapsulation of a Functionalized Ingredient in Alginate-Pectin Nano- and Microparticles Loaded with Distinct Natural Actives: Stability and Antioxidant Effect. Food Hydrocoll. 2020, 101, 105560. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Tiznado-Hernández, M.E.; Jha, D.K.; Janmeja, N.; Arul, J. Abiotic Stress Hormesis: An Approach to Maintain Quality, Extend Storability, and Enhance Phytochemicals on Fresh Produce during Postharvest. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3659–3682. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Yamauchi, M.; Sekiya, M.; Shimonishi, Y.; Tanaka, F. Responses of Phytonutrients and Tissue Condition in Persimmon and Cucumber to Postharvest UV-C Irradiation. Postharvest Biol. Technol. 2018, 145, 33–40. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Escalona, V.H.; Robles, P.A.; Martínez-Hernández, G.B.; Artés, F. Effect of UV-C Radiation on Quality of Minimally Processed Spinach Leaves. J. Sci. Food Agric. 2009, 89, 414–421. [Google Scholar] [CrossRef]
- Galindo-Pérez, M.J.; Quintanar-Guerrero, D.; Mercado-Silva, E.; Real-Sandoval, S.A.; Zambrano-Zaragoza, M.L. The Effects of Tocopherol Nanocapsules/Xanthan Gum Coatings on the Preservation of Fresh-Cut Apples: Evaluation of Phenol Metabolism. Food Bioprocess Technol. 2015, 8, 1791–1799. [Google Scholar] [CrossRef]
- Zhang, M.; Zhan, Z.G.; Wang, S.J.; Tang, J.M. Extending the Shelf-Life of Asparagus Spears with a Compressed Mix of Argon and Xenon Gases. LWT Food Sci. Technol. 2008, 41, 686–691. [Google Scholar] [CrossRef]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetables Products, 2nd ed.; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- González-Reza, R.M.; Hernandez-Sanchez, H.; Zambrano-Zaragoza, M.L.; Gutierrez-Lopez, G.F.; Del Real, A.; Quintanar-Guerrero, D.; Velasco-Bejarano, B. Influence of Stabilizing and Encapsulating Polymers on Antioxidant Capacity, Stability, and Kinetic Release of Thyme Essential Oil Nanocapsules. Foods 2020, 9, 1884. [Google Scholar] [CrossRef] [PubMed]
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity Effects on Enzymatic Browning and Antioxidant Capacity of Fresh-Cut Baby Romaine Lettuce (Lactuca sativa L. Cv. Duende). Food Chem. 2010, 119, 1502–1506. [Google Scholar] [CrossRef]
- García-Betanzos, C.I.; Hernández-Sánchez, H.; Bernal-Couoh, T.F.; Quintanar-Guerrero, D.; de la Luz Zambrano-Zaragoza, M. Physicochemical, Total Phenols and Pectin Methylesterase Changes on Quality Maintenance on Guava Fruit (Psidium guajava L.) Coated with Candeuba Wax Solid Lipid Nanoparticles-Xanthan Gum. Food Res. Int. 2017, 101, 218–227. [Google Scholar] [CrossRef]
- Pistone, S.; Qoragllu, D.; Smistad, G.; Hiorth, M. Formulation and Preparation of Stable Cross-Linked Alginate-Zinc Nanoparticles in the Presence of a Monovalent Salt. Soft Matter. 2015, 11, 5765–5774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonassen, H.; Treves, A.; Kjøniksen, A.L.; Smistad, G.; Hiorth, M. Preparation of Ionically Cross-Linked Pectin Nanoparticles in the Presence of Chlorides of Divalent and Monovalent Cations. Biomacromolecules 2013, 14, 3523–3531. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe Vera and Ascorbic Acid Coatings Maintain Postharvest Quality and Reduce Microbial Load of Strawberry Fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, M.A. Modified Atmosphere Packaging of Fresh-Cut Papaya Using Alginate Based Edible Coating: Quality Evaluation and Shelf Life Study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Park, J.J.; Lee, J.J.; Young, L.W. Combined Effect of Chitosan Coating and Modified Atmosphere Packaging on Fresh-Cut Cucumber. Food Sci. Nutr. 2019, 7, 1043–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istúriz-Zapata, M.A.; Hernández-López, M.; Correa-Pacheco, Z.N.; Barrera-Necha, L.L. Quality of Cold-Stored Cucumber as Affected by Nanostructured Coatings of Chitosan with Cinnamon Essential Oil and Cinnamaldehyde. LWT 2020, 123, 109089. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; del Real, L.A.; Gutiérrez-Cortez, E.; Cornejo-Villegas, M.A.; Quintanar-Guerrero, D. The Effect of Nano-Coatings with α-Tocopherol and Xanthan Gum on Shelf-Life and Browning Index of Fresh-Cut “Red Delicious” Apples. Innov. Food Sci. Emerg. Technol. 2014, 22, 188–196. [Google Scholar] [CrossRef]
- Kleinhenz, M.; Bumgarner, N. Using Brix as an indicator of vegetable quality. In Linking Measured Values to Crop Management. Fact Sheet. Agriculture and Natural Resources; The Ohio State University: Columbus, OH, USA, 2012. [Google Scholar]
- Meng, F.; Zhang, Y.; Xiong, Z.; Wang, G.; Li, F.; Zhang, L. Mechanical, Hydrophobic and Thermal Properties of an Organic-Inorganic Hybrid Carrageenan-Polyvinyl Alcohol Composite Film. Compos. Part B Eng. 2018, 143, 1–8. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Gómez, P.A.; Pradas, I.; Artés, F.; Artés-Hernández, F. Moderate UV-C Pretreatment as a Quality Enhancement Tool in Fresh-Cut Bimi® Broccoli. Postharvest Biol. Technol. 2011, 62, 327–337. [Google Scholar] [CrossRef]
- Vàsquez, H.; Ouhibi, C.; Lizzi, Y.; Azzouz, N.; Forges, M.; Bardin, M.; Nicot, P.; Urban, L.; Aarrouf, J. Pre-Harvest Hormetic Doses of UV-C Radiation Can Decrease Susceptibility of Lettuce Leaves (Lactuca sativa L.) to Botrytis cinerea L. Sci. Hortic. 2017, 222, 32–39. [Google Scholar] [CrossRef]
- Kasim, R.; Ufuk Kasim, M. UV-C Treatments on Fresh-Cut Garden Cress (Lepidium sativum L.) Enhanced Chlorophyll Content and Prevent Leaf Yellowing. World Appl. Sci. J. 2012, 17, 509–515. [Google Scholar]
- Artés-Hernández, F.; Robles, P.A.; Gómez, P.A.; Tomás-Callejas, A.; Artés, F. Low UV-C illumination for keeping overall quality of fresh-cut watermelon. Postharvest Biol. Technol. 2010, 55, 114–120. [Google Scholar] [CrossRef]
- Gómez, P.L.; Schenk, M.L.; Salvatori, D.M.; Alzamora, S.M. Potential of UV-C Light for Preservation of Cut Apples Fortified with Calcium: Assessment of Optical and Rheological Properties and Native Flora Dynamics. Food Bioprocess Technol. 2015, 8, 1890–1903. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Liao, J.; Jiang, A.; Xiu, Z.; Gaowa, S.; Guan, Y.; Yang, X.; Feng, K.; Liu, C. Effect of Atmospheric Cold Plasma Treatment on Antioxidant Activities and Reactive Oxygen Species Production in Postharvest Blueberries during Storage. J. Sci. Food Agric. 2020, 100, 5586–5595. [Google Scholar] [CrossRef]
- Adiletta, G.; di Matteo, M.; Petriccione, M. Multifunctional Role of Chitosan Edible Coatings on Antioxidant Systems in Fruit Crops: A Review. Int. J. Mol. Sci. 2021, 22, 2633. [Google Scholar] [CrossRef] [PubMed]
- Klangmuang, P.; Sothornvit, R. Active Hydroxypropyl Methylcellulose-Based Composite Coating Powder to Maintain the Quality of Fresh Mango. LWT Food Sci. Technol. 2018, 91, 541–548. [Google Scholar] [CrossRef]
- Sessa, M.; Ferrari, G.; Donsì, F. Novel Edible Coating Containing Essential Oil Nanoemulsions to Prolong the Shelf Life of Vegetable Products. Chem. Eng. Trans. 2015, 43, 55–60. [Google Scholar] [CrossRef]
- Pan, Y.G.; Zu, H. Effect of UV-C Radiation on the Quality of Fresh-Cut Pineapples. Proc. Eng. 2012, 37, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Manzocco, L.; Da Pieve, S.; Maifreni, M. Impact of UV-C Light on Safety and Quality of Fresh-Cut Melon. Innov. Food Sci. Emerg. Technol. 2011, 12, 13–17. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation Techniques for Compounds and Products with Antioxidant and Antimicrobial Activity—A Critical View. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef]
- Freitas, A.; Moldão-Martins, M.; Costa, H.S.; Albuquerque, T.G.; Valente, A.; Sanches-Silva, A. Effect of UV-C radiation on bioactive compounds of pineapple (Ananas comosus L. Merr.) by-products. J. Sci. Food Agric. 2015, 95, 44–52. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L. The Use of Controlled Postharvest Abiotic Stresses as a Tool for Enhancing the Nutraceutical Content and Adding-Value of Fresh Fruits and Vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Teoh, L.S.; Lasekan, O.; Adzahan, N.M.; Hashim, N. The Effect of Combinations of UV-C Exposure with Ascorbate and Calcium Chloride Dips on the Enzymatic Activities and Total Phenolic Content of Minimally Processed Yam Slices. Postharvest Biol. Technol. 2016, 120, 138–144. [Google Scholar] [CrossRef]
- Chen, A.-J.; Luo, W.; Luo, Y.-B.; Zhu, B.-Z. Combined treatment of ultraviolet-C and L. plantarum on Salmonella enteritidis and quality control of fresh-cut apple. J. Food Process. Preserv. 2018, 42, e13349. [Google Scholar] [CrossRef] [Green Version]
- Akbari, E.; Gholami, M.; Ghobadi, C. Shelf-Life and Quality Attributes in Fresh-Cut Pear Cv. Shahmive Treated with Different Kinds of Antioxidants. J. Food Sci. Technol. 2019, 56, 3998–4008. [Google Scholar] [CrossRef]
- Rivera-Pastrana, D.M.; Gardea, A.A.; Yahia, E.M.; Martínez-Téllez, M.A.; González-Aguilar, G.A. Effect of UV-C Irradiation and Low Temperature Storage on Bioactive Compounds, Antioxidant Enzymes and Radical Scavenging Activity of Papaya Fruit. J. Food Sci. Technol. 2014, 51, 3821–3829. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Kennedy, J.F.; Zhang, X.; Heng, Y.; Chen, W.; Chen, Z.; Wu, X.; Wu, X. Preparation of Alginate Oligosaccharide and Its Effects on Decay Control and Quality Maintenance of Harvested Kiwifruit. Carbohydr. Polym. 2020, 242, 116462. [Google Scholar] [CrossRef]
- Chisari, M.; Barbagallo, R.N.; Spagna, G.; Artes, F. Improving the Quality of Fresh-Cut Melon through Inactivation of Degradative Oxidase and Pectinase Enzymatic Activities by UV-C Treatment. Int. J. Food Sci. Technol. 2011, 46, 463–468. [Google Scholar] [CrossRef]
- Taştan, Ö.; Pataro, G.; Donsì, F.; Ferrari, G.; Baysal, T. Decontamination of Fresh-Cut Cucumber Slices by a Combination of a Modified Chitosan Coating Containing Carvacrol Nanoemulsions and Pulsed Light. Int. J. Food Microbiol. 2017, 260, 75–80. [Google Scholar] [CrossRef]





| Days | HPMC | Control | HPMC-NP-LEO-A-P | UV-C-HPMC | UV-C-Control | UV-C/HPMC-NP-A-P |
|---|---|---|---|---|---|---|
| SST | ||||||
| 0 | 3.39 ± 0.20 a,a | 3.32 ± 0.31 a,a | 2.92 ± 0.32 a,b | 3.16 ± 0.21 a,a | 3.40 ± 0.10 c,a | 2.56 ± 0.21 a,c |
| 3 | 3.27 ± 0.10 a,a | 3.26 ± 0.10 a,a | 3.47 ± 0.10 b,b | 2.96 ± 0.06 b,c | 3.60 ± 0.30 a,b | 3.43 ± 0.25 b,b |
| 6 | 3.19 ± 0.16 a,a | 3.16 ± 0.17 a,a | 3.57 ± 0.15 b,c | 3.24 ± 0.06 a,a | 3.40 ± 0.46 a,c | 3.43 ± 0.15 b,c |
| 9 | 3.82 ± 0.23 b,a | 3.13 ± 0.10 a,b | 3.42 ± 0.16 b,c | 2.83 ± 0.25 b,d | 3.60 ± 0.55 a,a | 3.40 ± 0.26 b,a |
| 12 | 3.25 ± 0.30 a,a | 3.17 ± 0.16 a,a | 3.23 ± 0.06 c | 3.30 ± 0.17 a,a | 3.54 ± 0.51 a,b | 3.23 ± 0.15 c,a |
| 15 | 3.16 ± 0.10 a,a | 2.96 ± 0.06 b,a | 3.43 ± 0.10 b,b | 3.09 ± 0.30 a,a | 3.83 ± 0.15 b,c | 3.03 ± 0.21 d,a |
| pH | ||||||
| 0 | 5.41 ± 0.20 a,a | 5.26 ± 0.06 a,b | 5.19 ± 0.01 a,b | 5.10 ± 0.07 a,b | 5.35 ± 0.11 a,c | 5.19 ± 0.01 a,b |
| 3 | 5.53 ± 0.13 a,a | 5.17 ± 0.05 b,b | 5.49 ± 0.02 b,a | 5.17 ± 0.02 a,b | 5.33 ± 0.10 a,c | 5.33 ± 0.05 b,c |
| 6 | 5.43 ± 0.12 a,a | 5.57 ± 0.35 c,a | 5.51 ± 0.05 b,a | 5.77 ± 0.03 b,b | 5.60 ± 0.07 b,b | 5.49 ± 0.02 b,a |
| 9 | 5.59 ± 0.24 a,a | 6.24 ± 0.32 d,b | 5.48 ± 0.02 b,a | 5.61 ± 0.09 c,a | 6.29 ± 0.09 c,b | 5.54 ± 0.03 b,a |
| 12 | 6.12 ± 0.19 b,a | 6.68 ± 0.08 e,b | 5.57 ± 0.02 c,c | 5.71 ± 0.12 b,c | 6.39 ± 0.17 c,d | 5.71 ± 0.10 c,c |
| 15 | 6.42 ± 0.12 c,a | 6.74 ± 0.11 e,b | 5.59 ± 0.14 c,c | 5.76 ± 0.07 b,c | 6.37 ± 0.14 c,a | 5.79 ± 0.13 c,c |
| Days | Control | HPMC | HPMC-LEO-A-P-NP | UV-C Control | UV-C HPMC | UV-C HPMC- LEO-A-P-NP |
|---|---|---|---|---|---|---|
| 0 | 0.0 ± 0.0 a,a | 0.0 ± 0.0 a,a | 0.0 ± 0.0 a,a | 0.0 ± 0.0 a,a | 0.0 ± 0.0 a,a | 0.0 ± 0.0 a,a |
| 3 | 5.1 ± 0.6 b,a | 1.8 ± 0.8 b,b | 2.1 ± 0.8 b,c | 3.8 ± 0.9 b,d | 3.9 ± 0.5 b,d | 0.8 ± 0.2 b,e |
| 6 | 9.2 ± 1.2 c,a | 6.4 ± 0.8 c,a | 2.6 ± 1.0 b,b | 7.5 ± 1.2 c,c | 5.6 ± 0.9 c,b | 1.8 ± 0.5 c,d |
| 9 | 21.6 ± 1.4 d,a | 13.8 ± 1.2 d,b | 8.3 ± 1.2 d,c | 8.3 ± 1.3 c,c | 7.8 ± 0.8 d,d | 2.7 ± 0.3 d,e |
| 12 | 22.8 ± 1.3 d,a | 14.8 ± 2.9 d,b | 14.3 ± 1.8 e,b | 11.8 ± 1.5 d,c | 11.8 ± 1.7 e,c | 4.1 ± 0.5 e,d |
| 15 | 26.3 ± 2.3 d,a | 21.3 ± 4.3 e,a | 17.8 ± 1.6 f,b | 27.5 ± 3.6 e,a | 20.4 ± 2.1 f,b | 7.12 ± 1.2 f,c |
| Days | HPMC | Control | HPMC- LEO-A-P-NP | HPMC-UV-C | Control-UV-C | UV-C-HPMC- LEO-A-P-NP |
|---|---|---|---|---|---|---|
| Total Phenols (mg EGA/g of fresh product) | ||||||
| 0 | 5.83 ± 0.52 a,a | 5.79 ± 0.19 a,a | 6.36 ± 0.15 a,b | 7.44 ± 0.04 a,c | 7.12 ± 0.16 a,d | 7.80 ± 0.03 a,f |
| 3 | 5.49 ± 0.24 b,a | 5.31 ± 0.18 b,b | 6.58 ± 0.20 a,c | 5.55 ± 0.06 b,a | 4.74 ± 0.44 b, | 7.12 ± 0.08 b, |
| 6 | 5.12 ± 0.34 b,a | 2.69 ± 0.08 c,b | 6.24 ± 0.10 a,c | 5.14 ± 0.10 c,a | 3.93 ± 0.03 c,d | 6.26 ± 0.09 c,c |
| 9 | 3.87 ± 0.65 c,a | 2.41 ± 0.09 c,b | 5.15 ± 0.13 b,c | 4.25 ± 0.19 d,d | 2.30 ± 0.22 d,b | 5.68 ± 0.09 d,e |
| 12 | 5.78 ± 0.28 a,a | 4.96 ± 0.21 d,b | 4.88 ± 0.12 b,b | 5.35 ± 0.04 e,c | 5.14 ± 0.07 e,d | 5.62 ± 0.18 d,a |
| 15 | 3.60 ± 0.99 c,a | 5.01 ± 0.23 e,b | 7.08 ± 0.03 c,c | 6.73 ± 0.83 f,d | 6.24 ± 0.59 f,d | 4.77 ± 0.42 e,b |
| DPPH μmol TE/100 g fresh product | ||||||
| 0 | 535 ± 8 a,a | 530 ± 12 a,a | 567 ± 11 a,b | 692 ± 4 a,c | 575 ± 17 a,b | 657 ± 10 a,d |
| 3 | 404 ± 16 b,a | 399 ± 3 b,a | 407 ± 6 b,a | 486 ± 2 b,b | 415 ± 6 b,a | 470 ± 47 b,b |
| 6 | 327 ± 16 c,a | 243 ± 23 c,b | 385 ± 25 c,c | 339 ± 16 c,a | 265 ± 15 c,b | 466 ± 17 b,d |
| 9 | 328 ± 20 c,a | 206 ± 8 c,b | 405 ± 14 b,c | 326 ± 26 c,a | 232 ± 41 c,b | 482 ± 13 b,d |
| 12 | 247 ± 33 d,a | 335 ± 20 d,b | 427 ± 11 d,c | 391 ± 18 d,d | 209 ± 47 c,e | 450 ± 16 b,f |
| 15 | 460 ± 34 e,a | 386 ± 42 b,b | 386 ± 51 c,b | 326 ± 17 c,d | 418 ± 2 b,e | 427 ± 3 d,f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Leyva-Gómez, G.; Urbán-Morlán, Z. Effects of UV-C and Edible Nano-Coating as a Combined Strategy to Preserve Fresh-Cut Cucumber. Polymers 2021, 13, 3705. https://doi.org/10.3390/polym13213705
Zambrano-Zaragoza ML, Quintanar-Guerrero D, González-Reza RM, Cornejo-Villegas MA, Leyva-Gómez G, Urbán-Morlán Z. Effects of UV-C and Edible Nano-Coating as a Combined Strategy to Preserve Fresh-Cut Cucumber. Polymers. 2021; 13(21):3705. https://doi.org/10.3390/polym13213705
Chicago/Turabian StyleZambrano-Zaragoza, María L., David Quintanar-Guerrero, Ricardo M. González-Reza, María A. Cornejo-Villegas, Gerardo Leyva-Gómez, and Zaida Urbán-Morlán. 2021. "Effects of UV-C and Edible Nano-Coating as a Combined Strategy to Preserve Fresh-Cut Cucumber" Polymers 13, no. 21: 3705. https://doi.org/10.3390/polym13213705