In Situ Functionalization of Iron Oxide Particles with Alginate: A Promising Biosorbent for Retention of Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of Biosorbent (Alg-Fe3O4-MPs)
2.3. Biosorption Experiments
2.4. Equilibrium and Kinetic Models
2.5. Desorption Experiments
3. Results and Discussion
3.1. Characterization of Alg-Fe3O4-MPs Biosorbent
3.2. Biosorptive Performances of Alg-Fe3O4-MPs
3.2.1. Selection of Optimal Conditions
3.2.2. Isotherm Modelling
3.2.3. Adsorption Thermodynamics
3.2.4. Kinetics Modelling
3.3. Biosorption Mechanism
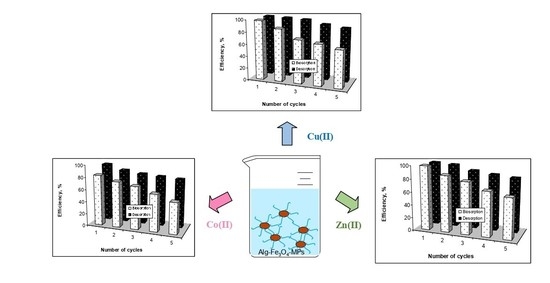
3.4. Desorption and Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akpor, O.B.; Muchie, M. Environmental and public health implications of wastewater quality. Afr. J. Biotechnol. 2011, 10, 2379–2387. [Google Scholar]
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Atkovska, K.; Lisichkov, K.; Ruseska, G.; Dimitrov, A.T.; Grozdanov, A. Removal of heavy metal ions from wastewater using conventional and nanosorbents: A review. J. Chem. Technol. Metall. 2018, 53, 202–219. [Google Scholar]
- Bose, P.; Aparna Bose, M.; Kumar, S. Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc and cyanide. Adv. Environ. Res. 2002, 7, 179–195. [Google Scholar] [CrossRef]
- Aji, B.A.; Yavuz, Y.; Koparal, A.S. Electrocoagulation of heavy metals containing model wastewater using monopolar ion electronedes. Sep. Purif. Technol. 2012, 86, 248–254. [Google Scholar] [CrossRef]
- Edebali, S.; Pehlivan, E. Evalution of chelate and cation exchange resins to remove copper ions. Powder Tehnol. 2016, 301, 520–525. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Balasubramanian, R. Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cano, L.A.; García-Rosero, H.; Gonzalez-Gutierrez, L.V.; Baldenegro-Pérez, L.A.; Carrasco-Marín, F. Functionalized adsorbents prepared from fruit peels: Equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. J. Clean. Prod. 2017, 162, 195–204. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athat, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Biores. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- El-Sayed, S.; Hyun-Seog, R.; Subhabrata, D.; Moonis, A.K.; Abou-Shanab, R.A.I.; Chang, S.W.; Jeon, B.H. Algae as a green technology for heavy metals removal from various wastewater. World J. Microbiol. Biotechnol. 2019, 35, 75–94. [Google Scholar]
- Deniz, F.; Karabulut, A. Biosorption of heavy metal ions by chemically modified biomass of coastal seaweed community: Studies on phycoremediation system modeling and design. Ecol. Eng. 2017, 106, 101–108. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.L.; Lau, B.F.; Chang, J.S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Lucaci, A.R.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Equilibrium and Kinetics Studies of Metal Ions Biosorption on Alginate Extracted from Marine Red Algae Biomass (Callithamnion corymbosum sp.). Polymers 2020, 12, 1888. [Google Scholar] [CrossRef]
- Chee, S.Y.; Wong, P.K.; Wong, C.L. Extraction and characterisation of alginate from brown seaweeds (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. J. Appl. Phycol. 2011, 23, 191–219. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. A Comparison of Palladium Sorption Using Polyethylenimine Impregnated Alginate-Based and Carrageenan-Based Algal Beads. Appl. Sci. 2018, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Vauchel, P.; Leroux, K.; Kaas, R.; Arhaliass, A.; Baron, R.; Legrand, J. Kinetics modeling of alginate alkaline extraction from Laminaria digitata. Bioresour. Technol. 2009, 100, 1291–1296. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; Rivers, A.; Stuckey, D.C.; Ward, K. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohydr. Polym. 2020, 245, 116419. [Google Scholar] [CrossRef] [PubMed]
- Lucaci, A.R.; Bulgariu, L. Comparative study of alginate extraction methods from marine algae Callithamnion Corymbosum Sp. Bull. Polytech. Inst. Jassy 2018, 64, 51–60. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intra-particle diffusion processes during acid dye adsorption onto chitosan. Biores. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Chong, K.H.; Volesky, B. Description of two-metal biosorption equilibria by Langmuir-type models. Biotechnol. Bioeng. 1995, 47, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangabhashiyam, S.; Anu, N.; Nandagopal Giri, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural by-products. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Fresenius, W.; Quentin, K.E.; Schneider, W. Water Analysis. A Practical Guide to Physico-Chemical, Chemical and Microbiological Water Examination and Quality Assurance; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Qu, J.; Meng, X.; You, H.; Ye, X.; Du, Z. Utilization of rice husks functionalized with xanthates as cost-effective biosorbents for optimal Cd (II) removal from aqueous solution via response surface methodology. Biores. Technol. 2017, 241, 1036–1042. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, L.K.; Gaur, J.P. Metal sorption by algal biomass: From batch to continuous system. Algal Res. 2016, 18, 95–109. [Google Scholar] [CrossRef]
- Dean, J.A. Handbook of Analytical Chemistry; Mc-Grow Hill Inc.: New York, NY, USA, 1995. [Google Scholar]
- Kumar, Y.P.; King, P.; Prasad, V.S.R.K. Removal of copper from aqueous solution using Ulva fasciata sp. A marine green algae. J. Hazard. Mater. 2006, 137, 367–373. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Gęca, M.; Skwarek, E.; Goncharuk, O. Titania-coated silica alone and modified by sodium alginate as sorbents for heavy metal ions. Nanoscale Res. Lett. 2018, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, J.; Li, Z.; Fan, L.; Chen, R.; Wu, X.; Li, J.; Zeng, W. A high-efficiency Fe2O3@Microalgae composite for heavy metal removal from aqueous solution. J. Water Proc. Eng. 2020, 33, 101026. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhat, A.H.; Buang, A. Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: Kinetic and equilibrium modeling. J. Clean. Prod. 2018, 171, 1361–1375. [Google Scholar] [CrossRef]










| Isotherm Parameter | Cu(II) | Co(II) | Zn(II) | |
|---|---|---|---|---|
| Langmuir model | R2 | 0.9819 | 0.9877 | 0.9851 |
| qmax, mmol/g | 0.7262 | 0.3842 | 0.6528 | |
| KL, L/mmol | 2.8151 | 5.3866 | 3.6127 | |
| Freundlich model | R2 | 0.9302 | 0.9089 | 0.9481 |
| n | 3.73 | 1.88 | 2.34 | |
| KF, L1/n/g·mmol1/(n−1) | 0.7369 | 0.2835 | 0.5927 | |
| Temkin model | R2 | 0.9599 | 0.9695 | 0.9002 |
| AT, L/g | 0.2852 | 0.3761 | 0.3847 | |
| B, kJ/mol | 20.82 | 17.46 | 19.14 | |
| Adsorbent | Cu(II) | Co(II) | Zn(II) | References |
|---|---|---|---|---|
| Ulva fasciata sp. | 0.42 | - | 0.21 | [35] |
| Callithamnion corymbosum sp. | 0.38 | 0.17 | 0.29 | [17] |
| Calcium-alginate | 1.02 | 0.32 | 0.56 | [17] |
| Alginate/polyethylenimine | 1.14 | - | 0.70 | [36] |
| Titania-coated silica/alginate | 0.32 | - | 0.27 | [37] |
| Fe2O3@Microalgae | 0.61 | - | - | [38] |
| Commercial Fe2O3 | 0.16 | - | - | [38] |
| Alg-Fe3O4-MPs | 0.73 | 0.38 | 0.65 | This study |
| Metal ion | ΔG0, kJ/mol | ΔH0, kJ/mol | ΔS0, J/mol K |
|---|---|---|---|
| Cu(II) | −42.03 | 15.57 | 192.43 |
| Co(II) | −42.39 | 14.03 | 189.05 |
| Zn(II) | −53.46 | 14.89 | 228.85 |
| Kinetics Parameter | Cu(II) | Co(II) | Zn(II) | ||
|---|---|---|---|---|---|
| qe,exp, mmol/g | 0.1624 | 0.1141 | 0.1344 | ||
| Pseudo-first order | R2 | 0.9132 | 0.9639 | 0.9539 | |
| qe,calc mmol/g | 0.0509 | 0.0408 | 0.0430 | ||
| k1, 1/min | 0.0130 | 0.0098 | 0.0143 | ||
| Pseudo-second order | R2 | 0.9999 | 0.9995 | 0.9999 | |
| qe,calc mmol/g | 0.1661 | 0.1167 | 0.1372 | ||
| k2, g/mmol min | 1.5223 | 1.5843 | 1.9794 | ||
| Intra-particle diffusion model | Zone 1 | R2 | 0.9516 | 0.8485 | 0.9573 |
| c, mmol/L | 0.0540 | 0.0468 | 0.0676 | ||
| kdiff1, mmol/g min1/2 | 0.0181 | 0.0097 | 0.0097 | ||
| Zone 2 | R2 | 0.9921 | 0.9663 | 0.9856 | |
| c, mmol/L | 0.1496 | 0.0892 | 0.1266 | ||
| kdiff2, mmol/g min1/2 | 0.0010 | 0.0019 | 0.0006 | ||
| Before Biosorption, Alg-Fe3O4-MPs | After Biosorption | ||
|---|---|---|---|
| Cu(II) | Co(II) | Zn(II) | |
| 3426.6 cm−1 | 3427.66 cm−1 | 3427.66 cm−1 | 3429.72 cm−1 |
| 2964.74 cm−1 | 2962.68 cm−1 | 2962.68 cm−1 | 2964.74 cm−1 |
| 2927.71 cm−1 | 2931.82 cm−1 | 2927.71 cm−1 | 2929.77 cm−1 |
| 2514.17 cm−1 | 2514.17 cm−1 | 2516.22 cm−1 | 2516.22 cm−1 |
| 1796.13 cm−1 | 1796.13 cm−1 | 1796.13 cm−1 | 1796.13 cm−1 |
| 1415.15 cm−1 | 1417.56 cm−1 | 1417.56 cm−1 | 1419.62 cm−1 |
| 1080.15 cm−1 | 1084.26 cm−1 | 1080.15 cm−1 | 1080.15 cm−1 |
| 874.40 cm−1 | 874.40 cm−1 | 874.40 cm−1 | 874.40 cm−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucaci, A.-R.; Bulgariu, D.; Bulgariu, L. In Situ Functionalization of Iron Oxide Particles with Alginate: A Promising Biosorbent for Retention of Metal Ions. Polymers 2021, 13, 3554. https://doi.org/10.3390/polym13203554
Lucaci A-R, Bulgariu D, Bulgariu L. In Situ Functionalization of Iron Oxide Particles with Alginate: A Promising Biosorbent for Retention of Metal Ions. Polymers. 2021; 13(20):3554. https://doi.org/10.3390/polym13203554
Chicago/Turabian StyleLucaci, Alina-Roxana, Dumitru Bulgariu, and Laura Bulgariu. 2021. "In Situ Functionalization of Iron Oxide Particles with Alginate: A Promising Biosorbent for Retention of Metal Ions" Polymers 13, no. 20: 3554. https://doi.org/10.3390/polym13203554