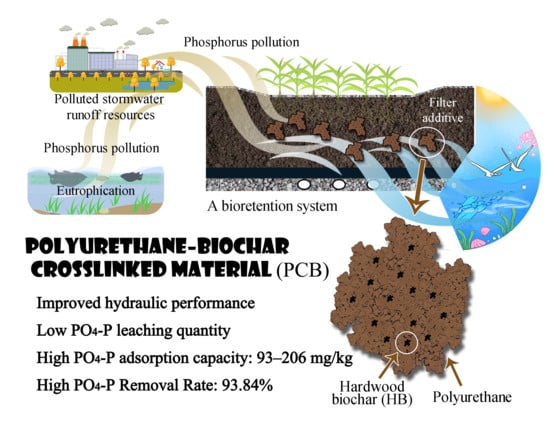
Phosphorus Release and Adsorption Properties of Polyurethane–Biochar Crosslinked Material as a Filter Additive in Bioretention Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Polyurethane–Biochar Crosslinked Material
- Hardwood biochar (HB) production: the raw material for the synthesis of PCB used in this study is commercially common hardwood biochar, which was produced using pine at a 600 ℃ pyrolysis temperature and was purchased from Jinlian Landscape Engineering Services Co., LTD. (Hangzhou, China).
- Polyurethane-biochar crosslinked material (PCB) preparation: PCB was synthesized with a simple one-shoot method, where the polyol and HB (for modifying polyurethane) were mixed with isocyanate. The polyol source used in this research was glycol and isocyanate was diphenyl-methane-diisocyanate (MDI).
- PCB production: 60 g of glycol, 100 g of deionized water (DW) and 5 g of HB were mixed continuously at 750 rpm and 60 °C for 20 min with a magnetic stirrer (VRera, Nanjing, China). After that, while keeping the same rotating speed and temperature, 250 g of MDI was added dropwise at a constant speed before air bubbles formed. The procedure was continued by pouring the mixture into a 30 × 30 × 10 cm3 of mold and transferring it to a vacuum oven (Xidebao, Shanghai, China) at 60 °C for 3 h and then curing it for 24 h.
- Cutting: The cured PCB was cut into granules with a particle size of 1–2mm, considering the practical application and consistent research scale of HB.
- The PCB used in this study was produced with the assistance of Jinlian Company. Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) were conducted on a Hitachi SU3500/S4800 High-Resolution Focused Ion Beam and Scanning Electron Microscope (Hitachi, Tokyo, Japan) working at an accelerating voltage of 10 kV, helping to illustrate the microstructure of PCB and HB.
2.2. The Hydraulic Properties and Other Physicochemical Characterizations Tests
- Saturated moisture content test: The natural bulk densities of the PCB and HB were measured by the cutting ring method (ISO 11272:2017). The samples in the cutting ring were vacuumed by a pump, immersed in deionized water (DW) for 24 h, weighed, dried in an oven at 60 °C for 48 h and weighed again to determine the natural and saturated moisture content (ISO 17892-1:2014).
- Permeability coefficient test: The permeability coefficient of the materials was determined by the constant head method with a Type 70 permeameter (Nanjing Soil Instrument Factory Co., LTD., Nanjing, China) (ISO 17892-11:2019).
- Other physicochemical characterizations influencing the leaching and adsorption capacity of materials were tested:
- The particle size of materials was measured by a sieving method.
- The specific gravity of the materials was measured by the gravity bottle method and the pore ratio of the materials was obtained after conversion with the saturated water content.
- The pH of modifier materials was measured at a material/DW ratio of 1:50 by mass.
- BET surface area was determined by N2 (77 K) adsorption on an ASAP 2020 Accelerated Surface Area and Porosimetry System (Micromeritics Instrument, Atlanta, GA, USA) after degassing for 12 h via VacPrepTM 061 (Micromeritics Instrument, Atlanta, GA, USA), a Gas Adsorption Sample Preparation Device.
- The cation exchange capacity (CEC) was determined by a hexamminecobalt (III) chloride solution (ISO 23470:2018).
- TP (total phosphorus content) of materials was measured after strong acid digestion and analyzed by ICP-OES (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Leaching Experiments
2.4. Phosphate Adsorption Experiments
2.5. Column Experiments
3. Results and Discussion
3.1. Polymerization Process and Microstructure
3.2. Hydraulic Properties of Modifiers
3.3. Phosphorus Leaching
3.4. Leaching of Other Ions
3.5. Phosphate Adsorption
3.6. Stormwater Infiltration Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beretta-Blanco, A.; Carrasco-Letelier, L. Relevant factors in the eutrophication of the Uruguay River and the Río Negro. Sci. Total Environ. 2020, 143299. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint Pollution of Surface Waters with Phosphorus and Nitrogen. Ecol. Appl. 1998, 8, 559. [Google Scholar] [CrossRef]
- Laurenson, G.; Laurenson, S.; Bolan, N.; Beecham, S.; Clark, I. The Role of Bioretention Systems in the Treatment of Stormwater. Adv. Agron. 2013, 120, 223–274. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Davis, A.P. Evaluation and Optimization of Bioretention Media for Treatment of Urban Storm Water Runoff. J. Environ. Eng. 2005, 131, 1521–1531. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.P.; Shokouhian, M.; Sharma, H.; Minami, C. Laboratory study of biological retention for urban stormwater management. Water Environ. Res. 2001, 73, 5–14. [Google Scholar] [CrossRef]
- Hunt, W.F.; Smith, J.T.; Jadlocki, S.J.; Hathaway, J.M.; Eubanks, P.R. Pollutant Removal and Peak Flow Mitigation by a Bioretention Cell in Urban Charlotte, N.C. J. Environ. Eng. 2008, 134, 403–408. [Google Scholar] [CrossRef]
- Tirpak, R.A.; Afrooz, A.N.; Winston, R.J.; Valenca, R.; Schiff, K.; Mohanty, S. Conventional and amended bioretention soil media for targeted pollutant treatment: A critical review to guide the state of the practice. Water Res. 2021, 189, 116648. [Google Scholar] [CrossRef]
- Li, L.; Davis, A.P. Urban Stormwater Runoff Nitrogen Composition and Fate in Bioretention Systems. Environ. Sci. Technol. 2014, 48, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Apaolaza, L.; Guerrero, F. Comparison between pine bark and coconut husk sorption capacity of metals and nitrate when mixed with sewage sludge. Bioresour. Technol. 2008, 99, 1544–1548. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, A.P.S.; Firmino, M.A.; Nomura, C.S.; Rocha, F.R.P.; Oliveira, P.V.; Gaubeur, I. Peat as a natural solid-phase for copper preconcentration and determination in a multicommuted flow system coupled to flame atomic absorption spectrometry. Anal. Chim. Acta 2009, 636, 198–204. [Google Scholar] [CrossRef]
- Li, P.; Lang, M.; Li, C.; Thomas, B.W.; Hao, X. Nutrient Leaching from Soil Amended with Manure and Compost from Cattle Fed Diets Containing Wheat Dried Distillers’ Grains with Solubles. Water, Air, Soil Pollut. 2016, 227, 393. [Google Scholar] [CrossRef]
- Jiang, C.; Li, J.; Li, H.; Li, Y. An improved approach to design bioretention system media. Ecol. Eng. 2019, 136, 125–133. [Google Scholar] [CrossRef]
- Cai, M.; Li, F.C.; Chen, S.H.; Gao, L.; Guo, L. The adsorption effect of three minerals on chemical oxygen demand, total nitrogen, total phosphorus and heavy metals in biogas slurry. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 042034. [Google Scholar] [CrossRef]
- Lin, H.; Ma, R.; Lin, J.; Sun, S.; Liu, X.; Zhang, P. Positive effects of zeolite powder on aerobic granulation: Nitrogen and phosphorus removal and insights into the interaction mechanisms. Environ. Res. 2020, 191, 110098. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Miller, V.; Chiu, P.C.; Maresca, J.A.; Guo, M.; Imhoff, P.T. Nutrient release and ammonium sorption by poultry litter and wood biochars in stormwater treatment. Sci. Total. Environ. 2016, 553, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.A. The Charcoal Vision: A Win–Win–Win Scenario for Simultaneously Producing Bioenergy, Permanently Sequestering Carbon, while Improving Soil and Water Quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef] [Green Version]
- Spokas, K.A.; Novak, J.M.; Masiello, C.A.; Johnson, M.G.; Colosky, E.C.; Ippolito, J.A.; Trigo, C. Physical Disintegration of Biochar: An Overlooked Process. Environ. Sci. Technol. Lett. 2014, 1, 326–332. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; You, C. Biochar Effect on Water Evaporation and Hydraulic Conductivity in Sandy Soil. Pedosphere 2016, 26, 265–272. [Google Scholar] [CrossRef]
- Ding, Y.; Deng, M.; Dong, J.; Wang, Z.; Huang, S. Relationship between Pore Structure and Thermal Prop-erties of Polyurethane under Multi-Field Coupling. Eng. Plast. Appl. 2019, 47, 90–94. (In Chinese) [Google Scholar]
- Kwiecień, K.; Kwiecień, A.; Stryszewska, T.; Szumera, M.; Dudek, M. Durability of PS-Polyurethane Dedicated for Composite Strengthening Applications in Masonry and Concrete Structures. Polym. 2020, 12, 2830. [Google Scholar] [CrossRef] [PubMed]
- Iqhrammullah, M.; Marlina, M.; Hedwig, R.; Karnadi, I.; Lahna, K.; Olaiya, N.G.; Haafiz, M.K.M.; Hps, A.K.; Abdulmadjid, S.N. Filler-Modified Castor Oil-Based Polyurethane Foam for the Removal of Aqueous Heavy Metals Detected Using Laser-Induced Breakdown Spectroscopy (LIBS) Technique. Polym. 2020, 12, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, Z.; Kim, K.-P.; Shin, J. Kinetic, thermodynamic and equilibrium studies for adsorption of ammonium ion on modified polyurethane. DESALINATION Water Treat. 2015, 57, 1–9. [Google Scholar] [CrossRef]
- Solares, S.B.; Merillas, B.; Cimavilla-Román, P.; Rodriguez-Perez, M.; Pinto, J. Enhanced nitrates-polluted water remediation by polyurethane/sepiolite cellular nanocomposites. J. Clean. Prod. 2020, 254, 120038. [Google Scholar] [CrossRef]
- Okoli, C.P.; Adewuyi, G.O.; Zhang, Q.; Diagboya, P.N.; Qingjun, G. Mechanism of dialkyl phthalates removal from aqueous solution using γ-cyclodextrin and starch based polyurethane polymer adsorbents. Carbohydr. Polym. 2014, 114, 440–449. [Google Scholar] [CrossRef] [Green Version]
- Anju, M.; Renuka, N. Magnetically actuated graphene coated polyurethane foam as potential sorbent for oils and organics. Arab. J. Chem. 2020, 13, 1752–1762. [Google Scholar] [CrossRef]
- Lu, H.; Tian, H.; Zhang, M.; Zhang, M.; Chen, Q.; Guan, R.; Wang, H. Water Polishing improved controlled-release characteristics and fertilizer efficiency of castor oil-based polyurethane coated diammonium phosphate. Sci. Rep. 2020, 10, 5763. [Google Scholar] [CrossRef] [Green Version]
- Yong, W.; Jie, L. Use of Polyurethane Foams Complex Material to Preserve Moisture and Fertility. Polym. Technol. Eng. 2007, 46, 943–947. [Google Scholar] [CrossRef]
- Sasidharan, A.P.; Meera, V.; Raphael, V.P. Investigations on characteristics of polyurethane foam impregnated with na-nochitosan and nanosilver/silver oxide and its effectiveness in phosphate removal. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Nie, J.Y.; Zhu, N.; Lin, K.M.; Song, F.Y. Effect of soil fortified by polyurethane foam on septic tank effluent treatment. Water Sci. Technol. 2011, 63, 1230–1235. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Zhang, X.; Silva, L.C.R.; Six, J.; Parikh, S.J. Use of Chemical and Physical Characteristics To Investigate Trends in Biochar Feedstocks. J. Agric. Food Chem. 2013, 61, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total. Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.; Mahmoud, D.; Ahmad, A. Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (Cocos nucifera) bunch waste. J. Hazard. Mater. 2008, 158, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Teodosiu, C.; Wenkert, R.; Tofan, L.; Paduraru, C. Advances in preconcentration/removal of environmentally relevant heavy metal ions from water and wastewater by sorbents based on polyurethane foam. Rev. Chem. Eng. 2014, 30, 403–420. [Google Scholar] [CrossRef]
- Korley, L.T.J.; Pate, B.D.; Thomas, E.L.; Hammond, P.T. Effect of the degree of soft and hard segment ordering on the morphology and mechanical behavior of semicrystalline segmented polyurethanes. Polym. 2006, 47, 3073–3082. [Google Scholar] [CrossRef]
- Tan, Z.; Yuan, S.; Hong, M.; Zhang, L.; Huang, Q. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef]
- Motawie, A.; Madani, M.; Esmail, E.; Dacrorry, A.; Othman, H.; Badr, M.; Abulyazied, D. Electrophysical characteristics of polyurethane/organo-bentonite nanocomposites. Egypt. J. Pet. 2014, 23, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Zhou, Z.; Peng, C.; Liu, W.; Zhou, H.; Wang, W.; Zhang, Q. Influence of Mass Ratio of Polyols on Properties of Polycaprolactone- Polyethylene Glycol/Methylene Diphenyl Diisocyanate/Diethylene Glycol Hydrogels. J. Macromol. Sci. Part B 2017, 944, 315–323. [Google Scholar] [CrossRef]
- Yang, Y.; Tong, Z.; Geng, Y.; Li, Y.; Zhang, M. Biobased Polymer Composites Derived from Corn Stover and Feather Meals as Double-Coating Materials for Controlled-Release and Water-Retention Urea Fertilizers. J. Agric. Food Chem. 2013, 61, 8166–8174. [Google Scholar] [CrossRef]
- New Jersey Stormwater Best Management Practices Manual. 2007. Available online: https://www.njstormwater.org/bmp_manual2.htm (accessed on 16 January 2021).
- De Macedo, M.B.; Lago, C.A.F.D.; Mendiondo, E.M. Stormwater volume reduction and water quality improvement by bioretention: Potentials and challenges for water security in a subtropical catchment. Sci. Total. Environ. 2019, 647, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Chunyan, J.; Shunli, H.; Shusheng, G.; Wei, X.; Huaxun, L.; Yuhai, Z. The Characteristics of Lognormal Distribution of Pore and Throat Size of a Low Permeability Core. Pet. Sci. Technol. 2013, 31, 856–865. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Chiu, P.C.; Imhoff, P.T.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total. Environ. 2015, 512–513, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Stubbings, W.A.; Harrad, S. Leaching of TCIPP from furniture foam is rapid and substantial. Chemosphere 2018, 193, 720–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef] [PubMed]
- Angst, T.E.; Sohi, S.P. Establishing release dynamics for plant nutrients from biochar. GCB Bioenergy 2012, 5, 221–226. [Google Scholar] [CrossRef]
- Shin, H.; Tiwari, D.; Kim, D.-J. Phosphate adsorption/desorption kinetics and P bioavailability of Mg-biochar from ground coffee waste. J. Water Process. Eng. 2020, 37, 101484. [Google Scholar] [CrossRef]
- Gupta, N.; Gedam, V.V.; Moghe, C.; Labhasetwar, P. Comparative assessment of batch and column leaching studies for heavy metals release from Coal Fly Ash Bricks and Clay Bricks. Environ. Technol. Innov. 2019, 16, 100461. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. The role of biochar porosity and surface functionality in augmenting hydrologic properties of a sandy soil. Sci. Total. Environ. 2017, 574, 139–147. [Google Scholar] [CrossRef]
- Brzeska, J.; Tercjak, A.; Sikorska, W.; Kowalczuk, M.; Rutkowska, M. Predicted Studies of Branched and Cross-Linked Polyurethanes Based on Polyhydroxybutyrate with Polycaprolactone Triol in Soft Segments. Polymers 2020, 12, 1068. [Google Scholar] [CrossRef]
- Akkas, T.; Citak, C.; Sirkecioglu, A.; Guner, F.S. Which is more effective for protein adsorption: Surface roughness, surface wettability or swelling? Case study of polyurethane films prepared from castor oil and poly(ethylene glycol). Polym. Int. 2013, 62, 1202–1209. [Google Scholar] [CrossRef]








| Media Material | ρ1 (g/cm3) | Particle Size (mm) | e2 | ωsat3 (%) | K4 (cm/s) | pH | BET (m2/g) | CEC (cmol/kg) | TP 5 (g/kg) |
|---|---|---|---|---|---|---|---|---|---|
| PCB | 0.165 | 1–2 | 3.20 | 383.50% | 8.56 × 10−2 | 6.62 | 83.14 | 37.5 | 1.19 |
| HB | 0.378 | <0.5 | 3.88 | 195.65% | 6.57 × 10−4 | 8.80 | 118.45 | 7.4 | 3.80 |
| Media Material | PO4-P | TP-P | |||||
|---|---|---|---|---|---|---|---|
| 8 rounds (μmol/g) | 1 round (μmol/g) | 1 round/ 8-rounds | 8 rounds (μmol/g) | 8 rounds/ Total | 1 round (μmol/g) | 1 round/ 8 rounds | |
| PCB-DW | 2.68 | 1.47 | 54.85% | 9.16 | 23.86% | 4.09 | 44.65% |
| HB-DW | 7.11 | 0.19 | 2.67% | 8.55 | 6.98% | 0.27 | 3.16% |
| PCB-AS | −4.82 1 | −0.82 1 | - | 0.38 | - | 1.81 | - |
| HB-AS | −13.67 1 | −1.79 1 | - | −11.51 1 | - | −1.53 1 | - |
| Media Material | Na+ | K+ | Mg2+ | Ca2+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 rounds (μmol/g) | 1 round (μmol/g) | 1 round/ 8 rounds | 8 rounds (μmol/g) | 1 round (μmol/g) | 1 round/ 8 rounds | 8 rounds (μmol/g) | 1 round (μmol/g) | 1 round/ 8-rounds | 8 rounds (μmol/g) | 1 round (μmol/g) | 1 round/ 8 rounds | |
| PCB-DW | 4.28 | 3.13 | 73.05% | 16.78 | 8.67 | 51.69% | 25.48 | 5.92 | 23.23% | 23.27 | 4.71 | 20.22% |
| HB-DW | 3.91 | 1.10 | 28.04% | 90.18 | 39.16 | 43.42% | 20.55 | 4.29 | 20.90% | 83.56 | 12.39 | 14.83% |
| PCB-AS | −1.53 | −0.72 | - | 12.07 | 5.39 | 44.66% | 42.59 | 4.33 | 10.17% | −50.65 | −12.45 | - |
| HB-AS | 28.04 | 19.87 | - | 254.03 | 190.68 | 75.06% | 48.45 | 18.15 | 37.46% | 83.16 | 55.63 | - |
| Elements | PCB | PCB-DW | HB | HB-DW |
|---|---|---|---|---|
| Wt% | ||||
| C | 58.74 | 55.89 | 38.16 | 31.93 |
| O | 35.18 | 37.64 | 32.04 | 38.17 |
| Na | - | - | 0.62 | 0.27 |
| Mg | 0.83 | 0.73 | 0.74 | - |
| Al | 0.83 | 0.31 | 1.56 | 0.37 |
| Si | 1.26 | 0.24 | 17.61 | 25.96 |
| K | 0.64 | - | 3.16 | 0.86 |
| Ca | 2.52 | 5.19 | 6.11 | 2.44 |
| Total | 100 | |||
| Material | Freundlich | Langmuir | |||||
|---|---|---|---|---|---|---|---|
| KF (L/mg) | 1/n | R2 | qmax (mg/kg) | KL (L/mg) | R2 | RL | |
| PCB-DW-DW | 214.978 | 0.460 | 0.998 | 417.833 | 1.3156 | 0.959 | 0.071–0.603 |
| HB-DW-DW | 80.500 | 0.623 | 0.983 | 374.176 | 0.274 | 0.994 | 0.267–0.880 |
| PCB-DW-AS | 186.782 | 0.467 | 0.990 | 379.160 | 0.510 | 0.960 | 0.164–0.797 |
| HB-DW-AS | 69.599 | 0.606 | 0.977 | 319.12 | 0.276 | 0.990 | 0.266–0.879 |
| Columns | Concentration of the First 50 mL Effluent (mg/L) | Total Leaching Quantities (mg) | ||
|---|---|---|---|---|
| Predicted | Detected | Predicted | Detected | |
| PCB-Column | 47.60 | 0.80 | 52.07 | 1.22 |
| HB-Column | 8.20 | 0.03 | 184.03 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Wang, Y.; Wang, C. Phosphorus Release and Adsorption Properties of Polyurethane–Biochar Crosslinked Material as a Filter Additive in Bioretention Systems. Polymers 2021, 13, 283. https://doi.org/10.3390/polym13020283
Meng Y, Wang Y, Wang C. Phosphorus Release and Adsorption Properties of Polyurethane–Biochar Crosslinked Material as a Filter Additive in Bioretention Systems. Polymers. 2021; 13(2):283. https://doi.org/10.3390/polym13020283
Chicago/Turabian StyleMeng, Yike, Yuan Wang, and Chuanyue Wang. 2021. "Phosphorus Release and Adsorption Properties of Polyurethane–Biochar Crosslinked Material as a Filter Additive in Bioretention Systems" Polymers 13, no. 2: 283. https://doi.org/10.3390/polym13020283