An Estimate of the Onset of Beadless Character of Electrospun Nanofibers Using Rheological Characterization
Abstract
:1. Introduction
- (a)
- copolymer of poly(vinylidene fluoride) and hexafluoropropylene (PVDF-co-HFP) dissolved in N,N’-dimethylformamide (DMF),
- (b)
- poly(ethylene oxide) (PEO) dissolved in water, and
- (c)
- poly(vinyl butyral) (PVB) dissolved in ethanol.
2. Materials and Methods
2.1. Material
2.2. Preparation of Electrospinning Solution
2.3. Process of Electrospinning
2.4. Rheological Measurements
2.5. Characterization of Nanofibrous Mats
3. Results
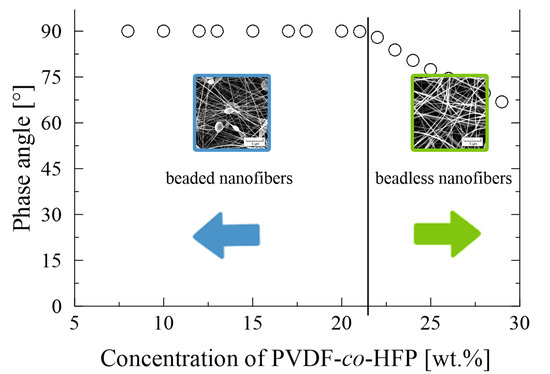
3.1. PVDF-co-HFP Nanofibres
3.2. PVB Nanofibres
3.3. PEO Nanofibres
3.4. Summary of the Individual Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Q.; Li, Y.; Yang, M. Polyaniline nanofiber humidity sensor prepared by electrospinning. Sens. Actuators B Chem. 2012, 161, 967–972. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Highly sensitive and ultrafast response surface acoustic wave humidity sensor based on electrospun polyaniline/poly(vinyl butyral) nanofibers. Anal. Chim. Acta 2012, 748, 73–80. [Google Scholar] [CrossRef]
- McKee, M.G.; Wilkes, G.L.; Colby, R.H.; Long, T.E. Correlations of solution rheology with electrospun fiber formation of linear and branched polyesters. Macromolecules 2004, 37, 1760–1767. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer-polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, A. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doğan, Y.K.; Demirural, A.; Baykara, T. Single-needle electrospinning of PVA hollow nanofibers for core–shell structures. SN Appl. Sci. 2019, 1, 415. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; He, J.H.; Yu, J.Y.; Zeng, H.M. Controlling numbers and sizes of beads in electrospun nanofibers. Polym. Int. 2008, 57, 632–636. [Google Scholar] [CrossRef]
- Habeeb, S.A.; Rajabi, L.; Dabirian, F. Production of polyacrylonitrile/boehmite nanofibrous composite tubular structures by opposite-charge electrospinning with enhanced properties from a low-concentration polymer solution. Polym. Compos. 2020, 41, 1649–1661. [Google Scholar] [CrossRef]
- Josef, E.; Guterman, R. Designing solutions for electrospinning of poly(ionic liquid)s. Macromolecules 2019, 52, 5223–5230. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-7248-3. [Google Scholar]
- Hsu, H.H.; Chiu, Y.J.; Li, J.W.; Tseng, H.F.; Chang, K.C.; Chen, J.T. Alignment-improved and diameter-reduced electrospun polymer fibers via the hot-stretching process. Macromol. Mater. Eng. 2020, 305, 1900637. [Google Scholar] [CrossRef]
- de Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979; ISBN 080141203X. [Google Scholar]
- Filip, P.; Zelenkova, J.; Peer, P. Evaluation of an onset of electrospun beadless poly(ethylene oxide) nanofibres. J. Appl. Polym. Sci. 2020, 138, e50001. [Google Scholar] [CrossRef]
- Tsou, S.-Y.; Lin, H.-S.; Wang, C. Rheological aspect on electrospinning of polyamide 6 solutions. Polymer 2011, 52, 3127. [Google Scholar] [CrossRef]
- Chisca, S.; Barzic, A.I.; Sava, I.; Olaru, N.; Bruma, M. Morphological and rheological insights on polyimide chain entanglements for electrospinning produced fibers. J. Phys. Chem. B 2012, 116, 9082. [Google Scholar] [CrossRef]
- Wang, C.; Lee, M.-F.; Wu, Y.-J. Solution-electrospun poly(ethylene terephthalate) fibers: Processing and characterization. Macromolecules 2012, 45, 7939–7947. [Google Scholar] [CrossRef]
- Desitti, C.; Beliavski, M.; Tarre, S.; Avrahami, R.; Zussman, E.; Green, M. Durable electrospun microtubes for encapsulation of bacteria in atrazine bioremediation. J. Water Process. Eng. 2017, 19, 205–211. [Google Scholar] [CrossRef]
- Ahmed, F.; Choudhury, N.R.; Dutta, N.K.; Zannettino, A.; Knott, R. Near superhydrophobic fibrous scaffold for endothelialization: Fabrication, characterization and cellular activities. Biomacromolecules 2013, 14, 3850–3860. [Google Scholar] [CrossRef]
- Jung, H.-S.; Kim, M.H.; Shin, J.Y.; Park, S.R.; Jung, J.-Y.; Park, W.H. Electrospinning and wound healing activity of β-chitin extracted from cuttlefish bone. Carbohydr. Polym. 2018, 193, 205–211. [Google Scholar] [CrossRef]
- Kuntzler, S.G.; Vieira Costa, J.A.; de Morais, M.G. Development of electrospun nanofibers containing chitosan/PEO blend and phenolic compounds with antibacterial activity. Int. J. Biol. Macromol. 2018, 117, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.E.G.; Martínez, F.A.S.; Bossard, F.; Rinaudo, M. Biomaterials Based on Electrospun Chitosan. Relation between Processing Conditions and Mechanical Properties. Polymers 2018, 10, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, S.; Hussain, T.; Nazir, A.; Zahir, A.; Ramakrishna, S.; Hameed, M.; Khenoussi, N. Enhanced antibacterial activity of PEO-chitosan nanofibers with potential application in burn infection management. Int. J. Biol. Macromol. 2019, 135, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Vineis, C.; Varesano, A.; Mazzuchetti, G.; Ferrero, F.; Tonin, C. Structure and properties of keratin/PEO blend nanofibres. Europ. Polym. J. 2008, 44, 2465–2475. [Google Scholar] [CrossRef]
- Ma, H.; Shen, J.; Cao, J.; Wang, D.; Yue, B.; Mao, Z.; Wu, W.; Zhang, H. Fabrication of wool keratin/polyethylene oxide nano-membrane from wool fabric waste. J. Cleaner Prod. 2017, 161, 357–361. [Google Scholar] [CrossRef]
- Jin, H.J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning Bombyx mori silk with poly(ethylene oxide). Biomacromolecules 2002, 3, 1233–1239. [Google Scholar] [CrossRef]
- Wharram, S.E.; Zhang, X.; Kaplan, D.L.; McCarthy, S.P. Electrospun silk material systems for wound healing. Macromol. Biosci. 2010, 10, 246–257. [Google Scholar] [CrossRef]
- Hallensleben, M.L. Polyvinyl Compounds, Others. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Aftalion, F. A History of the International Chemical Industry, 2nd ed.; Chemical Heritage Press: Philadelphia, PA, USA, 2001; p. 153. ISBN 0-941901-29-7. [Google Scholar]
- Yalcinkaya, F. Experimental study on electrospun polyvinyl butyral nanofibers using a non-solvent system. Fiber Polym. 2015, 16, 2544–2551. [Google Scholar] [CrossRef]
- Mohammad Ali Zadeh, M.; Keyanpour-Rad, M.; Ebadzadeh, T. Synthesis of mullite nanofibres by electrospinning of solutions containing different proportions of polyvinyl butyral. Ceram. Int. 2013, 39, 9079–9084. [Google Scholar] [CrossRef]
- Campus. Available online: https://www.campusplastics.com/campus/en/datasheet/Kynar+Flex%C2%AE+2801-00/ARKEMA/179/5d7bdb8c (accessed on 14 January 2021).
- Kuraray. Available online: https://www.kuraray.com/products/mowital (accessed on 14 January 2021).
- Merck. Available online: https://www.sigmaaldrich.com/materials-science/material-science-products.html?TablePage=20204232 (accessed on 14 January 2021).
- Peer, P.; Stenicka, M.; Pavlinek, V.; Filip, P. The storage stability of polyvinylbutyral solutions from an electrospinnability standpoint. Polym. Degr. Stab. 2014, 105, 134–139. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Taylor cone and jetting from liquid droplets in electrospinning of nanofibers. J. Appl. Phys. 2001, 90, 4836–4846. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.H.; Fridrikh, S.V.; Rutledge, G.C. The role of elasticity in the formation of electrospun fibers. Polymer 2006, 47, 4789–4797. [Google Scholar] [CrossRef]
- Suresh, S.; Becker, A.; Glasmacher, B. Impact of apparatus orientation and gravity in electrospinning—A review of empirical evidence. Polymers 2020, 12, 2448. [Google Scholar] [CrossRef] [PubMed]
- Nain, A.K. Densities, ultrasonic speeds, viscosities and excess properties of binary mixtures of methyl methacrylate with N,N-dimethylformamide and N,Ndimethylacetamide at different temperatures. J. Chem. Thermodyn. 2013, 60, 105–116. [Google Scholar] [CrossRef]
- Rowley, R.L.; Wilding, W.V.; Knotts, T.A., IV; Giles, N. DIPPR Project 801 Data Compilation of Pure Compound Properties; AIChE Design Institute for Physical Properties: New York, NY, USA, 2017. [Google Scholar]
- Gonçalves, F.A.M.M.; Trindade, A.R.; Costa, C.S.M.F.; Bernardo, J.C.S.; Johnson, I.; Fonseca, I.M.A.; Ferreira, A.G.M. PVT, viscosity, and surface tension of ethanol: New measurements and literature data evaluation. J. Chem. Thermodyn. 2010, 42, 1039–1049. [Google Scholar] [CrossRef]
- Dortmund Data Bank. Available online: http://www.ddbst.com/en/EED/PCP/VIS_C11.php (accessed on 14 January 2021).
- Japanese Standards Association (JSA), JIS Z 8803. Available online: https://www.jsa.or.jp/en/ (accessed on 14 January 2021).
- Filip, P.; Peer, P.; Zelenkova, J. Dependence of poly(vinyl butyral) electrospun fibres diameter on molecular weight and concentration. J. Ind. Text. 2020. accepted. [Google Scholar] [CrossRef]
- Filip, P.; Peer, P. Characterization of poly(ethylene oxide) nanofibres—Mutual relations between mean diameter of electrospun nanofibres and solution characteristics. Processes 2019, 7, 948. [Google Scholar] [CrossRef] [Green Version]
- Zatloukal, M.; Kopytko, W.; Vlcek, J. The effect of different batches of the same polymer on the flow in flat coextrusion dies. In Proceedings of the Annual Technical Conference of the Society of Plastics Engineers, Nashville, TN, USA, 4–8 May 2003; pp. 3509–3513. [Google Scholar]








| Material | Molecular Weight (g/mol) | Concentration (%) |
|---|---|---|
| PVDF-co-HFP (solvent N,N′-dimethylformamide) | 455,000 a | 8, 10, 12, 13, 15, 17, 18, 20–29 in steps of 1% |
| PVB (solvent ethanol) | 75,000 a | 3–10 in steps of 1%, 12, 14 |
| PEO (solvent distilled water) | 637,500 b | 0.3, 0.5, 0.6, 0.8, 1, 1.2, 1.5, 1.8, 2—6 in steps of 0.5%, 7, 7.5, 8, 9 |
| Material | Overlap Concentration c* (wt.%) | Entanglement Concentration ce* (wt.%) | Starting Concentration cstart (wt.%) |
|---|---|---|---|
| PVDF-co-HFP (solvent DMF) | 12 | 19 | 22 |
| PVB (solvent ethanol) | 5 | 8 | 7.5 |
| PEO (solvent distilled water) | 1.4 | 4.6 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peer, P.; Zelenkova, J.; Filip, P.; Lovecka, L. An Estimate of the Onset of Beadless Character of Electrospun Nanofibers Using Rheological Characterization. Polymers 2021, 13, 265. https://doi.org/10.3390/polym13020265
Peer P, Zelenkova J, Filip P, Lovecka L. An Estimate of the Onset of Beadless Character of Electrospun Nanofibers Using Rheological Characterization. Polymers. 2021; 13(2):265. https://doi.org/10.3390/polym13020265
Chicago/Turabian StylePeer, Petra, Jana Zelenkova, Petr Filip, and Lenka Lovecka. 2021. "An Estimate of the Onset of Beadless Character of Electrospun Nanofibers Using Rheological Characterization" Polymers 13, no. 2: 265. https://doi.org/10.3390/polym13020265