Esterification of Alginate with Alkyl Bromides of Different Carbon Chain Lengths via the Bimolecular Nucleophilic Substitution Reaction: Synthesis, Characterization, and Controlled Release Performance
Abstract
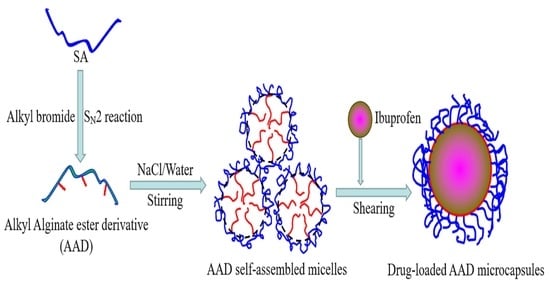
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Homogeneous Synthesis of AAD with Different Hydrophobic Side Groups
2.3. Characterization of AAD
2.4. Self-Aggregation Performance of AAD
2.5. Preparation of the Drug-Loaded AAD Microcapsules and Its Release Performance
2.6. Cytotoxicity of AAD
3. Results and Discussion
3.1. Synthesis and Characterization of AAD
3.2. Colloidal Interface Activity of AAD
3.3. Drug-Loading and Release Performance of AAD Microcapsules
3.4. Cytocompatibility of AAD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Liu, R.; Liu, W.; Kang, H. Synthesis, self-assembly, and thermosensitive properties of ethyl cellulose-g-P (PEGMA) amphiphilic copolymers. J. Polym. Sci. Pol. Chem. 2008, 46, 6907–6915. [Google Scholar] [CrossRef]
- Yuan, W.; Li, X.; Gu, S.; Cao, A.; Ren, J. Amphiphilic chitosan graft copolymer via combination of ROP, ATRP and click chemistry: Synthesis, self-assembly, thermosensitivity, fluorescence, and controlled drug release. Polymer 2011, 52, 658–666. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Synthesis and characterization of a novel amphiphilic chitosan–polylactide graft copolymer. Carbohydr. Polym. 2005, 59, 165–171. [Google Scholar] [CrossRef]
- Yang, J.S.; Zhou, Q.Q.; He, W. Amphipathicity and self-assembly behavior of amphiphilic alginate esters. Carbohydr. Polym. 2013, 92, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.Q.; Chen, X.Q.; Li, J.C.; Feng, Y.H.; Shi, Z.F.; Wang, X.H.; Lin, Q. Synthesis of alginate derivative via the Ugi reaction and its characterization. Carbohydr. Polym. 2016, 136, 757–763. [Google Scholar] [CrossRef]
- Yang, J.M.; Wang, N.C.; Chiu, H.C. Preparation and characterization of poly (vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer electrolytes membrane. J. Membr. Sci. 2014, 457, 139–148. [Google Scholar] [CrossRef]
- Yue, Y.; Han, J.; Han, G.; French, A.D.; Qi, Y.; Wu, Q. Cellulose nanofibers reinforced sodium alginate-polyvinyl alcohol hydrogels: Core-shell structure formation and property characterization. Carbohydr. Polym. 2016, 147, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Cacciotti, I.; Ceci, C.; Bianco, A.; Pistritto, G. Neuro-differentiated Ntera2 cancer stem cells encapsulated in alginate beads: First evidence of biological functionality. Mat. Sci. Eng. C 2017, 81, 32–38. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Tomren, H.B.; Christensen, B.E. Periodate oxidized alginates: Depolymerization kinetics. Carbohydr. Polym. 2011, 86, 1595–1601. [Google Scholar] [CrossRef]
- Bu, H.; Kjøniksen, A.L.; Elgsaeter, A.; Nyström, B. Interaction of unmodified and hydrophobically modified alginate with sodium dodecyl sulfate in dilute aqueous solution Calorimetric, rheological, and turbidity studies. Colloid. Surf. A 2006, 278, 166–174. [Google Scholar] [CrossRef]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Depan, D.; Kumar, A.P.; Singh, R.P. Cell proliferation and controlled drug release studies of nanohybrids based on chitosan-g-lactic acid and montmorillonite. Acta Biomater. 2009, 5, 93–100. [Google Scholar] [CrossRef]
- Nie, H.R.; He, A.H.; Zheng, J.F.; Xu, S.S.; Li, J.X.; Han, C.C. Effects of chain conformation and entanglement on the electrospinning of pure alginate. Biomacromolecules 2008, 9, 1362–1365. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.G.; Huang, Z.H.; Xue, F.F. Preparation and characterization of nanoparticles based on hydrophobic alginate derivative as carriers forsustained release of vitamin D3. J. Agric. Food Chem. 2011, 59, 1962–1967. [Google Scholar] [CrossRef]
- Vallée, F.; Müller, C.; Durand, A.; Schimchowitsch, S.; Dellacherie, E.; Kelche, C.; Cassel, J.C.; Leonard, M. Synthesis and rheological properties of hydrogels based onamphiphilic alginate-amide derivatives. Carbohydr. Res. 2009, 344, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2011, 33, 3279–3305. [Google Scholar] [CrossRef]
- Yang, J.S.; Ren, H.B.; Xie, Y.J. Synthesis of amidic alginate derivatives and their application in microencapsulation of λ-cyhalothrin. Biomacromolecules 2011, 12, 2982–2987. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Zhang, B.F.; Wen, L.Q.; Liang, Q.Y.; Zhang, L.M. Amphiphiliccholesteryl grafted sodium alginate derivative: Synthesis and self-assembly inaqueous solution. Carbohydr. Polym. 2007, 68, 218–225. [Google Scholar] [CrossRef]
- Kang, H.A.; Shin, M.S.; Yang, J.W. Preparation and characterization of hydrophobically modified alginate. Polym. Bull. 2002, 47, 429–435. [Google Scholar] [CrossRef]
- Fang, X.Q.; Zhao, X.Y.; Yu, G.B.; Zhang, L.; Feng, Y.H.; Zhou, Y.; Liu, Y.Y.; Li, J.C. Effect of molecular weight and pH on the self-assembly microstructural and emulsification of amphiphilic sodium alginate colloid particles. Food Hydrocolloid. 2020, 103, 105593. [Google Scholar] [CrossRef]
- Schweiger, R.G. Acetylation of alginic acid. II. Reaction of algin acetates with calcium and other divalent ions. J. Org. Chem. 1962, 27, 1789–1791. [Google Scholar] [CrossRef]
- Babak, V.G.; Skotnikova, E.A.; Lukina, I.G.; Pelletier, S.; Hubert, P.; Dellacherie, E. Hydrophobically associating alginate derivatives: Surface tension properties of their mixed aqueous solutions with oppositely charged surfactants. J. Colloid Interf. Sci. 2000, 225, 505–510. [Google Scholar] [CrossRef]
- Pelletier, S.; Hubert, P.; Lapicque, F.; Payan, E.; Dellacherie, E. Amphiphilic derivatives of sodium alginate and hyaluronate: Synthesis and physico-chemical properties of aqueous dilute solutions. Carbohydr. Polym. 2000, 43, 343–349. [Google Scholar] [CrossRef]
- Rastello De Boisseson, M.; Leonard, M.; Hubert, P.; Marchal, P.; Stequert, A.; Castel, C.; Favre, E.; Dellacherie, E. Physical alginate hydrogels based on hydrophobic or dual hydrophobic/ionic interactions: Bead formation, structure, and stability. J. Colloid. Interf. Sci. 2004, 273, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Chemical modification of alginates in organic solvent systems. Biomacromolecules 2011, 12, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate esters via chemoselective carboxyl group modification. Carbohydr. Polym. 2013, 98, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.Q.; Chen, X.Q.; Feng, M.X.; Shi, Z.F.; Zhang, W.; Wang, Y.; Ke, C.R.; Lin, Q. Entrapment of bacterial cellulose nanocrystals stabilized Pickering emulsions droplets in alginate beads for hydrophobic drug delivery. Colloid. Surface B 2019, 177, 112–120. [Google Scholar] [CrossRef]
- Yan, H.Q.; Chen, X.Q.; Bao, C.L.; Yi, J.L.; Lei, M.Y.; Ke, C.R.; Zhang, W.; Lin, Q. Synthesis and assessment of CTAB and NPE modified organomontmorillonite for the fabrication of organo-montmorillonite/alginate based hydrophobic pharmaceutical controlled-release formulation. Colloid. Surface B 2020, 191, 110983. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.; Bikiaris, D.; Avgoustakis, K.; Karavas, E.; Georgarakis, M. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr. Polym. 2008, 73, 44–54. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Erim, F.B. Alginate/BSA/montmorillonite composites with enhanced protein entrapment and controlled release efficiency. React. Funct. Polym. 2013, 73, 1420–1425. [Google Scholar] [CrossRef]
- Chen, X.Q.; Yan, H.Q.; Sun, W.; Feng, Y.H.; Li, J.C.; Lin, Q.; Shi, Z.F.; Wang, X.H. Synthesis of amphiphilic alginate derivatives and electrospinning blend nanofibers: A novel hydrophobic drug carrier. Polym. Bull. 2015, 72, 3097–3117. [Google Scholar] [CrossRef]
- Islam, M.S.; Karim, M.R. Fabrication and characterization of poly (vinylalcohol)/alginate blend nanofibers by electrospinning method. Colloid Surface A 2010, 366, 135–140. [Google Scholar] [CrossRef]
- Leonard, M.; Boisseson, M.R.D.; Hubert, P.; Dalencon, F.; Dellacherie, E. Hydrophobically modified alginate hydrogels as protein carriers with specific controlled release properties. J. Control. Release 2004, 98, 395–405. [Google Scholar] [CrossRef]
- Ionita, M.; Pandele, M.A.; Iovu, H. Sodium alginate/graphene oxidecomposite films with enhanced thermal and mechanical properties. Carbohydr. Polym. 2013, 94, 339–344. [Google Scholar] [CrossRef]
- Hua, S.B.; Ma, H.Z.; Li, X.; Yang, H.X.; Wang, A.Q. pH-sensitive sodium alginate/poly (vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Ji, L.; Yin, X.Q.; Zhu, L.; Lin, Q.; Qin, J.M. Synthesis and characterization of bacterial cellulose sulfates using a SO3/pyridine complex in DMAc/LiCl. Carbohydr. Polym. 2014, 101, 947–953. [Google Scholar] [CrossRef]
- Liu, C.G.; Desai, K.G.H.; Chen, X.G.; Park, H.J. Linolenic acid-modified chitosan for formation of self-assembled nanoparticles. J. Agric. Food Chem. 2005, 53, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Chen, X.G.; Park, H.J. Self-assembled nanoparticles based on linoleic-acid modified chitosan: Stability and absorption of trypsin. Carbohydr. Polym. 2005, 62, 293–298. [Google Scholar] [CrossRef]
- Goncalves, C.; Martins, J.A.; Gama, F.M. Self-Assembled nanoparticles of dextrin substituted with hexadecanethiol. Biomacromolecules 2007, 8, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, C.L.; Yang, Y.Q.; Zhu, Y.; Liu, N.; Liu, X.Y.; Luo, J.; Jiang, M. Self-assembly and emulsification of poly {[styrene-alt-maleic acid]-co-[styrene-alt-(N-3,4-dihydroxyphenylethyl-maleamic acid)]}. Langmuir 2012, 28, 9211–9222. [Google Scholar]
- Nie, H.R.; He, A.H.; Wu, W.L.; Zheng, J.F.; Xu, S.S.; Li, J.X.; Han, C.C. Effect of poly (ethylene oxide) with different molecular weights on the electrospinnability of sodium alginate. Polymer 2009, 50, 4926–4934. [Google Scholar] [CrossRef]
- An, Y.; Chen, M.; Xue, Q.; Liu, W. Preparation and self-assembly of carboxylic acid-functionalized silica. J. Colloid. Interf. Sci. 2007, 311, 507–513. [Google Scholar] [CrossRef]
- Hu, Z.; Ballinger, S.; Pelton, R.; Cranston, E.D. Surfactant-enhanced cellulose nanocrystal Pickering emulsions. J. Colloid. Interf. Sci. 2015, 439, 139–148. [Google Scholar] [CrossRef]
- Calabrese, I.; Cavallaro, G.; Lazzara, G.; Merli, M.; Sciascia, L.; Turco Liveri, M.L. Preparation and characterization of bio-organoclays using nonionic surfactant. Adsorption 2016, 22, 105116. [Google Scholar] [CrossRef]
- Calabrese, I.; Gelardi, G.; Merli, M.; Turco Liveri, M.L.; Sciascia, L. Clay-biosurfactant materials as functional drug delivery systems: Slowing down effect in the in vitro release of cinnamic acid. Appl. Clay Sci. 2017, 135, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.Q.; Chen, X.Q.; Shi, J.; Shi, Z.F.; Sun, W.; Lin, Q.; Wang, X.H.; Dai, Z.H. Fabrication and evaluation of chitosan/NaYF4:Yb3+/Tm3+ upconversion nanoparticles composite beads based on the gelling of Pickering emulsion droplets. Mat. Sci. Eng. C Mater. 2017, 71, 51–59. [Google Scholar] [CrossRef]











| Scheme | NAlkyl bromide/NHexuronic | TBAA (mmol) | Alkyl Bromide (mmol) | DS (%) | Yield (%) a |
|---|---|---|---|---|---|
| HAD | 0.3 | 2.59 | 0.78 | 26.2 | 76.8 |
| HAD | 0.5 | 2.59 | 1.30 | 44.6 | 79.5 |
| HAD | 1.2 | 2.59 | 3.11 | 100.0 | 83.4 |
| OAD | 0.5 | 2.59 | 1.30 | 42.3 | 78.4 |
| DAD | 0.5 | 2.59 | 1.30 | 41.5 | 79.7 |
| LAD | 0.5 | 2.59 | 1.30 | 40.1 | 78.6 |
| Sample | DS (%) | CAC Value (g/L) | Average dH (nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| HAD | 44.6 | 0.48 | 285.3 | −34.4 |
| OAD | 42.3 | 0.12 | 245.0 | −38.3 |
| DAD | 41.5 | 0.02 | 210.2 | −39.2 |
| LAD | 40.1 | 0.0068 | 180.5 | −44.8 |
| Formulation Code | EE | n | R2 | Diffusion Mechanism |
|---|---|---|---|---|
| SA microcapsules | 25.5% ± 3.2% | 0.9310 ± 0.0064 | 0.9815 | non-Fickian |
| HAD microcapsules | 71.6% ± 2.8% | 0.8524 ± 0.0061 | 0.9925 | non-Fickian |
| OAD microcapsules | 75.3% ± 3.0% | 0.8603 ± 0.0058 | 0.9930 | non-Fickian |
| DAD microcapsules | 77.1% ± 2.3% | 0.7990 ± 0.0057 | 0.9911 | non-Fickian |
| LAD microcapsules | 78.8% ± 2.5% | 0.8165 ± 0.0050 | 0.9904 | non-Fickian |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhu, Q.; Liu, C.; Li, D.; Yan, H.; Lin, Q. Esterification of Alginate with Alkyl Bromides of Different Carbon Chain Lengths via the Bimolecular Nucleophilic Substitution Reaction: Synthesis, Characterization, and Controlled Release Performance. Polymers 2021, 13, 3351. https://doi.org/10.3390/polym13193351
Chen X, Zhu Q, Liu C, Li D, Yan H, Lin Q. Esterification of Alginate with Alkyl Bromides of Different Carbon Chain Lengths via the Bimolecular Nucleophilic Substitution Reaction: Synthesis, Characterization, and Controlled Release Performance. Polymers. 2021; 13(19):3351. https://doi.org/10.3390/polym13193351
Chicago/Turabian StyleChen, Xiuqiong, Qingmei Zhu, Chang Liu, Dongze Li, Huiqiong Yan, and Qiang Lin. 2021. "Esterification of Alginate with Alkyl Bromides of Different Carbon Chain Lengths via the Bimolecular Nucleophilic Substitution Reaction: Synthesis, Characterization, and Controlled Release Performance" Polymers 13, no. 19: 3351. https://doi.org/10.3390/polym13193351