Comparison of Three Interfacial Conductive Networks Formed in Carbon Black-Filled PA6/PBT Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Composites
2.3. Characterization
3. Results and Discussion
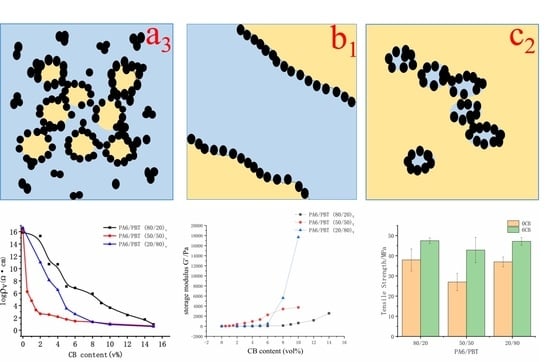
3.1. Electrical Percolation Behavior of CB in Three Different Blends
3.2. Comparison of the Composite Morphologies
3.3. Rheological Percolation Behavior of CB in Three Different Blends
3.4. Comparison of the Formation Mechanisms of Different Network Structures
3.5. Mechanical Properties of the Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, H.; Lin, L.; Ji, M.; Zhang, S.; Yang, M.; Fu, Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014, 39, 627–655. [Google Scholar] [CrossRef]
- Pang, H.; Xu, L.; Yan, D.-X.; Li, Z.-M. Conductive polymer composites with segregated structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- Mutiso, R.M.; Winey, K.I. Electrical properties of polymer nanocomposites containing rod-like nanofillers. Prog. Polym. Sci. 2015, 40, 63–84. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Ali Mohsin, M.E.; Shaikh, H.; Anis, A.; Pulose, A.M.; Yadav, M.K.; Qua, E.H.P.; Al-Zahrani, S.M. A review on electrically conductive polypropylene and polyethylene. Polym. Compos. 2014, 35, 900–914. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Su, Z.; Wei, G. Recent advances in the synthesis and applications of graphene–polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Yuan, T.; Sun, Z.; Mu, A.U.; Zeng, M.; Kalin, A.J.; Cheng, Z.; Olson, M.A.; Fang, L. Assembly and Chiral Memory Effects of Dynamic Macroscopic Supramolecular Helices. Chem. Eur. J. 2018, 24, 16553–16557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, H.; Liu, M.; Jin, Z.; Zhao, K. Effect of Core-Shell Morphology on the Mechanical Properties and Crystallization Behavior of HDPE/HDPE-g-MA/PA6 Ternary Blends. Polymers 2018, 10, 1040. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wang, J.; Zhang, B.-Y.; Guo, B.-H.; Yu, J.; Guo, Z.-X. Improved electrical conductivity of polymer/carbon black composites by simultaneous dispersion and interaction-induced network assembly. Compos. Sci. Technol. 2019, 179, 106–114. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, B.-Y.; Guo, B.-H.; Guo, Z.-X.; Yu, J. High-temperature polymer conductors with self-assembled conductive pathways. Compos. Part B Eng. 2020, 192, 107989. [Google Scholar] [CrossRef]
- Maiti, S.; Bera, R.; Karan, S.K.; Paria, S.; De, A.; Khatua, B.B. PVC bead assisted selective dispersion of MWCNT for designing efficient electromagnetic interference shielding PVC/MWCNT nanocomposite with very low percolation threshold. Compos. Part B Eng. 2019, 167, 377–386. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Zeng, L.; Li, R.; Tian, H.; Fu, X.; Wang, Y.; Zhong, W.-H. A review of the electrical and mechanical properties of carbon nanofiller-reinforced polymer composites. J. Mater. Sci. 2019, 54, 1036–1076. [Google Scholar] [CrossRef]
- Sumita, M.; Sakata, K.; Asai, S.; Keizo, M. Dispersion of fillers and the electrical conductivity of polymer blends filled with carbon black. Polym. Bull. 1991, 25, 265–271. [Google Scholar] [CrossRef]
- Duan, L.; Fu, S.; Deng, H.; Zhang, Q.; Wang, K.; Chen, F.; Fu, Q. The resistivity–strain behavior of conductive polymer composites: Stability and sensitivity. J. Mater. Chem. A 2014, 2, 17085–17098. [Google Scholar] [CrossRef]
- Zhang, S.; Deng, H.; Zhang, Q.; Fu, Q. Formation of Conductive Networks with Both Segregated and Double-Percolated Characteristic in Conductive Polymer Composites with Balanced Properties. ACS Appl. Mater. Interfaces 2014, 6, 6835–6844. [Google Scholar] [CrossRef] [PubMed]
- Bilotti, E.; Zhang, R.; Deng, H.; Baxendale, M.; Peijs, T. Fabrication and property prediction of conductive and strain sensing TPU/CNT nanocomposite fibres. J. Mater. Chem. 2010, 20, 9449–9455. [Google Scholar] [CrossRef]
- Bilotti, E.; Zhang, H.; Deng, H.; Zhang, R.; Fu, Q.; Peijs, T. Controlling the dynamic percolation of carbon nanotube based conductive polymer composites by addition of secondary nanofillers: The effect on electrical conductivity and tuneable sensing behaviour. Compos. Sci. Technol. 2013, 74, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Zonder, L.; Ophir, A.; Kenig, S.; McCarthy, S. The effect of carbon nanotubes on the rheology and electrical resistivity of polyamide 12/high density polyethylene blends. Polymer 2011, 52, 5085–5091. [Google Scholar] [CrossRef]
- Wang, X.; Xu, L.; Xu, X.-B.; Chen, S.; Wang, Y.-J. Effect of Mixing Process and Morphologies on the Electrical Conductivity of PA6/EVA/CB Composites. Polym. Plast. Technol. Eng. 2011, 50, 533–538. [Google Scholar] [CrossRef]
- Zhai, W.; Zhao, S.; Wang, Y.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Segregated conductive polymer composite with synergistically electrical and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2018, 105, 68–77. [Google Scholar] [CrossRef]
- Chen, J.; Cui, X.; Zhu, Y.; Jiang, W.; Sui, K. Design of superior conductive polymer composite with precisely controlling carbon nanotubes at the interface of a co-continuous polymer blend via a balance of π-π interactions and dipole-dipole interactions. Carbon 2017, 114, 441–448. [Google Scholar] [CrossRef]
- Shen, L.; Wang, F.; Jia, W.; Yang, H. Thermodynamically induced self-assembled electrically conductive networks in carbon-black-filled ternary polymer blends. Polym. Int. 2012, 61, 163–168. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. An innovative method to reduce percolation threshold of carbon black filled immiscible polymer blends. Compos. Part A Appl. Sci. Manuf. 2008, 39, 284–293. [Google Scholar] [CrossRef]
- Tan, Y.; Fang, L.; Xiao, J.; Song, Y.; Zheng, Q. Grafting of copolymers onto graphene by miniemulsion polymerization for conductive polymer composites: Improved electrical conductivity and compatibility induced by interfacial distribution of graphene. Polym. Chem. 2013, 4, 2939–2944. [Google Scholar] [CrossRef]
- Baudouin, A.-C.; Devaux, J.; Bailly, C. Localization of carbon nanotubes at the interface in blends of polyamide and ethylene–acrylate copolymer. Polymer 2010, 51, 1341–1354. [Google Scholar] [CrossRef]
- Baudouin, A.-C.; Auhl, D.; Tao, F.; Devaux, J.; Bailly, C. Polymer blend emulsion stabilization using carbon nanotubes interfacial confinement. Polymer 2011, 52, 149–156. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Yu, J.; Guo, Z.-X. Improved electrical conductivity of TPU/carbon black composites by addition of COPA and selective localization of carbon black at the interface of sea-island structured polymer blends. Soft Matter 2017, 13, 3431–3439. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Guo, B.-H.; Guo, Z.-X.; Yu, J. Conductive nylon-MXD6 composites prepared by melt compounding associated with formation of carbon black-covered PET domains serving as big conductive particles. Polymer 2019, 182, 121809. [Google Scholar] [CrossRef]
- Zha, X.-J.; Pu, J.-H.; Ma, L.-F.; Li, T.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. A particular interfacial strategy in PVDF/OBC/MWCNT nanocomposites for high dielectric performance and electromagnetic interference shielding. Compos. Part A Appl. Sci. Manuf. 2018, 105, 118–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, M.; Chen, P.; Yang, B.; Wu, B.; Miao, J.; Xia, R.; Qian, J. Improvement of the thermal/electrical conductivity of PA6/PVDF blends via selective MWCNTs-NH2 distribution at the interface. Mater. Des. 2019, 177, 107835. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.-K. Correlations between Percolation Threshold, Dispersion State, and Aspect Ratio of Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Jubinville, D.; Chang, B.P.; Pin, J.-M.; Mohanty, A.K.; Misra, M. Synergistic thermo-oxidative maleation of PA11 as compatibilization strategy for PA6 and PBT blend. Polymer 2019, 179, 121594. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; La Mantia, F.P.; Magagnini, P.; Acierno, D.; Gleria, M.; Bertani, R. Effect of adding new phosphazene compounds to poly(butylene terephthalate)/polyamide blends. I: Preliminary study in a batch mixer. Polym. Degrad. Stab. 2005, 90, 234–243. [Google Scholar] [CrossRef]
- Arboleda-Clemente, L.; Ares-Pernas, A.; García, X.; Dopico, S.; Abad, M.J. Segregated conductive network of MWCNT in PA12/PA6 composites: Electrical and rheological behavior. Polym. Compos. 2017, 38, 2679–2686. [Google Scholar] [CrossRef]
- Bitenieks, J.; Merijs Meri, R.; Zicans, J.; Buks, K. Dynamic Mechanical, Dielectrical, and Rheological Analysis of Polyethylene Terephthalate/Carbon Nanotube Nanocomposites Prepared by Melt Processing. Int. J. Polym. Sci. 2020, 2020, 5715463. [Google Scholar] [CrossRef]
- Jiang, C.; Han, S.; Chen, S.; Zhou, H.; Wang, X. The role of PTFE in-situ fibrillation on PET microcellular foaming. Polymer 2021, 212, 123171. [Google Scholar] [CrossRef]
- Hadaeghnia, M.; Ahmadi, S.; Ghasemi, I.; Wood-Adams, P.M. Manipulating the morphology of PA6/POE blends using graphene to achieve balanced electrical and mechanical properties. Compos. Sci. Technol. 2020, 200, 108412. [Google Scholar] [CrossRef]
- Gao, C.; Liu, P.; Ding, Y.; Li, T.; Wang, F.; Chen, J.; Zhang, S.; Li, Z.; Yang, M. Non-contact percolation of unstable graphene networks in poly(styrene-co-acrylonitrile) nanocomposites: Electrical and rheological properties. Compos. Sci. Technol. 2018, 155, 41–49. [Google Scholar] [CrossRef]
- Du, F.; Scogna, R.C.; Zhou, W.; Brand, S.; Fischer, J.E.; Winey, K.I. Nanotube Networks in Polymer Nanocomposites: Rheology and Electrical Conductivity. Macromolecules 2004, 37, 9048–9055. [Google Scholar] [CrossRef]
- Fernández, M.; Landa, M.; Muñoz, M.E.; Santamaria, A. Electrical conductivity of PUR/MWCNT nanocomposites in the molten state, during crystallization and in the solid state. Eur. Polym. J. 2011, 47, 2078–2086. [Google Scholar] [CrossRef]
- Canales, J.; Muñoz, M.E.; Fernández, M.; Santamaría, A. Rheology, electrical conductivity and crystallinity of a polyurethane/graphene composite: Implications for its use as a hot-melt adhesive. Compos. Part A Appl. Sci. Manuf. 2016, 84, 9–16. [Google Scholar] [CrossRef]
- Omurtag, P.S.; Alkan, B.; Durmaz, H.; Hizal, G.; Tunca, U. Indirect functionalization of multiwalled carbon nano tubes through non-covalent interaction of functional polyesters. Polymer 2018, 141, 213–220. [Google Scholar] [CrossRef]
- Hoseini, A.H.A.; Arjmand, M.; Sundararaj, U.; Trifkovic, M. Tunable electrical conductivity of polystyrene/polyamide-6/carbon nanotube blend nanocomposites via control of morphology and nanofiller localization. Eur. Polym. J. 2017, 95, 418–429. [Google Scholar] [CrossRef]
- Huang, J.-C. Carbon black filled conducting polymers and polymer blends. Adv. Polym. Technol. 2002, 21, 299–313. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, H.-B.; Hong, S.; Jiang, Z.-G.; Gui, C.; Li, X.; Yu, Z.-Z. Simultaneous Improvement in Both Electrical Conductivity and Toughness of Polyamide 6 Nanocomposites Filled with Elastomer and Carbon Black Particles. Ind. Eng. Chem. Res. 2014, 53, 2270–2276. [Google Scholar] [CrossRef]
- Mondal, S.; Ganguly, S.; Das, P.; Khastgir, D.; Das, N.C. Low percolation threshold and electromagnetic shielding effectiveness of nano-structured carbon based ethylene methyl acrylate nanocomposites. Compos. Part. B Eng. 2017, 119, 41–56. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Tuo, X.; Guo, B.-H.; Yu, J.; Guo, Z.-X. Comparison of Three Interfacial Conductive Networks Formed in Carbon Black-Filled PA6/PBT Blends. Polymers 2021, 13, 2926. https://doi.org/10.3390/polym13172926
Li H, Tuo X, Guo B-H, Yu J, Guo Z-X. Comparison of Three Interfacial Conductive Networks Formed in Carbon Black-Filled PA6/PBT Blends. Polymers. 2021; 13(17):2926. https://doi.org/10.3390/polym13172926
Chicago/Turabian StyleLi, Hansong, Xinlin Tuo, Bao-Hua Guo, Jian Yu, and Zhao-Xia Guo. 2021. "Comparison of Three Interfacial Conductive Networks Formed in Carbon Black-Filled PA6/PBT Blends" Polymers 13, no. 17: 2926. https://doi.org/10.3390/polym13172926