Expanded Polystyrene Beads Coated with Intumescent Flame Retardant Material to Achieve Fire Safety Standards
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of the Water-Based Intumescent Flame Retardant Formulation
2.3. Preparation of MDI-Coated EPS Beads
2.4. Preparation of Flame Retardant MDI-Coated EPS Foam
2.5. Characterizations
2.5.1. Combustion Behavior
2.5.2. Cone Calorimetry Test (CCT)
3. Results and Discussion
3.1. Chemical STRucture
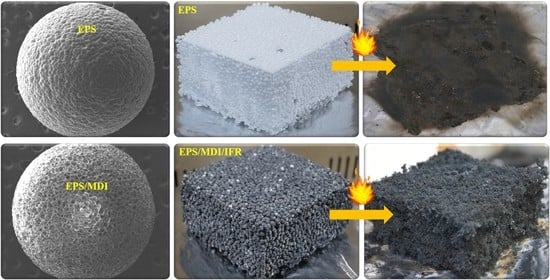
3.2. Microstructural Study
3.3. Mass Calculation
3.4. Thermogravimetric Analysis (TGA)
3.5. Combustion Behavior
3.6. Cone Calorimetry Analysis
3.7. Char Residue Analysis
Chemical Composition Char Residue
3.8. Possible Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ürge-Vorsatz, D.; Cabeza, L.F.; Serrano, S.; Barreneche, C.; Petrichenko, K. Heating and cooling energy trends and drivers in buildings. Renew. Sustain. Energy Rev. 2015, 41, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Di Foggia, G. Energy efficiency measures in buildings for achieving sustainable development goals. Heliyon 2018, 4, e00953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, M.A. The role of energy efficiency in sustainable development. Proceedings 1995 Interdisciplinary Conference: Knowledge Tools for a Sustainable Civilization. In Proceedings of the Fourth Canadian Conference on Foundations and Applications of General Science Theory, Toronto, ON, Canada, 8–10 June 1995; pp. 140–148. [Google Scholar] [CrossRef]
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A review of unconventional sustainable building insulation materials. Sustain. Mater. Technol. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Mourad, A.H.; Hittini, W.; Hassan, M.; Hameedi, S. Traditional, state-of-the-art and renewable thermal building insulation materials: An overview. Constr. Build. Mater. 2019, 214, 709–735. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, W.; Han, Y.; Gu, X.; Li, H.; Sun, J. Flame-retardant expandable polystyrene foams coated with ethanediol-modified melamine–formaldehyde resin and microencapsulated ammonium polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46471. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Liu, P.; Jing, Z.; Ge, X.; Jiang, Y. The flame resistance properties of expandable polystyrene foams coated with a cheap and effective barrier layer. Constr. Build. Mater. 2018, 176, 403–414. [Google Scholar] [CrossRef]
- Lu, H.; Wilkie, C.A. Study on intumescent flame retarded polystyrene composites with improved flame retardancy. Polym. Degrad. Stab. 2010, 95, 2388–2395. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, H.; Liu, N. Ignition of expandable polystyrene foam by a hot particle: An experimental and numerical study. J. Hazard. Mater. 2015, 283, 536–543. [Google Scholar] [CrossRef]
- Wang, G.; Chen, X.; Liu, P.; Bai, S. Flame-retardant mechanism of expandable polystyrene foam with a macromolecular nitrogen–phosphorus intumescent flame retardant. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Li, Y.; Shao, Q.; Yan, X.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame retardant. Polym. Adv. Technol. 2018, 29, 668–676. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, Y.; Yang, S.; Guo, Z.; Zhang, T.; Song, H.; Shao, Q. Esterification synthesis of ethyl oleate catalyzed by Brønsted acid-surfactant-combined ionic liquid. Green Chem. Lett. Rev. 2017, 10, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Liu, S.; Xiong, K.; Wang, W.; Liu, Y. Highly flame retardancy of cotton fabrics with a novel phosphorus/nitrogen/silicon flame-retardant treating system. Fibers Polym. 2016, 17, 569–575. [Google Scholar] [CrossRef]
- Jian, R.K.; Ai, Y.F.; Xia, L.; Zhao, L.J.; Zhao, H.B. Single component phosphamide-based intumescent flame retardant with potential reactivity towards low flammability and smoke epoxy resins. J. Hazard. Mater. 2019, 371, 529–539. [Google Scholar] [CrossRef]
- Li, M.E.; Wang, S.X.; Han, L.X.; Yuan, W.J.; Cheng, J.B.; Zhang, A.N.; Zhao, H.B.; Wang, Y.Z. Hierarchically porous SiO2/polyurethane foam composites towards excellent thermal insulating, flame-retardant and smoke-suppressant performances. J. Hazard. Mater. 2019, 375, 61–69. [Google Scholar] [CrossRef]
- Gijsman, P.; Steenbakkers, R.; Fürst, C.; Kersjes, J. Differences in the flame retardant mechanism of melamine cyanurate in polyamide 6 and polyamide 66. Polym. Degrad. Stab. 2002, 78, 219–224. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, S.; Liu, G. A THEIC-based polyphosphate melamine intumescent flame retardant and its flame retardancy properties for polylactide. J. Anal. Appl. Pyrolysis 2016, 122, 24–34. [Google Scholar] [CrossRef]
- Han, J.; Liang, G.; Gu, A.; Ye, J.; Zhang, Z.; Yuan, L. A novel inorganic-organic hybridized intumescent flame retardant and its super flame retarding cyanate ester resins. J. Mater. Chem. A 2013, 1, 2169–2182. [Google Scholar] [CrossRef]
- Jimenez, M.; Duquesne, S.; Bourbigot, S. Intumescent fire protective coating: Toward a better understanding of their mechanism of action. Thermochim. Acta 2006, 449, 16–26. [Google Scholar] [CrossRef]
- Tang, M.; Qi, F.; Chen, M.; Sun, Z.; Xu, Y.; Chen, X.; Zhang, Z.; Shen, R. Synergistic effects of ammonium polyphosphate and red phosphorus with expandable graphite on flammability and thermal properties of HDPE/EVA blends. Polym. Adv. Technol. 2016, 27, 52–60. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.T.; Wang, D.Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Li, Y.; Zou, J.; Zhou, S.; Chen, Y.; Zou, H.; Liang, M.; Luo, W. Effect of expandable graphite particle size on the flame retardant, mechanical, and thermal properties of water-blown semi-rigid polyurethane foam. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Zirnstein, B.; Schulze, D.; Scharte, B. Mechanical and fire properties of multicomponent flame retardant EPDM rubbers using aluminum trihydroxide, ammonium polyphosphate, and polyaniline. Materials 2019, 12, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Braun, U.; Schartel, B. Flame retardant mechanism of red phosphorus and magnesium hydroxide in high impact polystyrene. Macromol. Chem. Phy. 2004, 205, 2185–2196. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Han, Z. Enhanced flame retardancy of polyethylene/magnesium hydroxide with polycarbosilane. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, X.B.; Liu, X.L. Effect of magnesium hydroxide on the flame retardant properties of unsaturated polyester resin. Procedia Eng. 2013, 52, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Diani, J.; Gall, K. Finite Strain 3D Thermoviscoelastic Constitutive Model. Society 2006, 382, 1–10. [Google Scholar] [CrossRef]
- Hornsby, P.R. Fire retardant fillers for polymers. Int. Mater. Rev. 2001, 46, 199–210. [Google Scholar] [CrossRef]
- Pal, K.; Rastogi, J.N. Development of halogen-free flame-retardant thermoplastic elastomer polymer blend. J. Appl. Polym. Sci. 2004, 94, 407–415. [Google Scholar] [CrossRef]
- Wang, H.; Tian, J. Synergistic antiflaming of expandable graphite on the sealing silicone rubber. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 706–709. [Google Scholar] [CrossRef]
- Javangula, H.; Lineberry, Q. Comparative studies on fire-rated and standard gypsum wallboard. J. Therm. Anal. Calorim. 2014, 116, 1417–1433. [Google Scholar] [CrossRef]
- Pedersen, B.F.; Semmingsen, D. Neutron diffraction refinement of the structure of gypsum, CaSO4.2H2O. Acta Crystallogr. 1982, B38, 1074–1077. [Google Scholar] [CrossRef] [Green Version]
- Ballirano, P.; Melis, E. Thermal behaviour and kinetics of dehydration of gypsum in air from in situ real-time laboratory parallel-beam X-ray powder diffraction. Phys. Chem. Miner. 2009, 36, 391–402. [Google Scholar] [CrossRef]
- Dorigato, A.; Fredi, G.; Fambri, L.; Lopez-Cuesta, J.M.; Pegoretti, A. Polyethylene-based single polymer laminates: Synergistic effects of nanosilica and metal hydroxides. J. Reinf. Plast. Compos. 2019, 38, 62–73. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L.; Chen, H.; Jia, H.; Tang, W. Effect of chicken eggshell on the flame-retardant and smoke suppression properties of an epoxy-based traditional APP-PER-MEL system. Polym. Compos. 2019, 40, 2712–2723. [Google Scholar] [CrossRef]
- Rajaei, M.; Wang, D.Y.; Bhattacharyya, D. Combined effects of ammonium polyphosphate and talc on the fire and mechanical properties of epoxy/glass fabric composites. Compos. Part B Eng. 2017, 113, 381–390. [Google Scholar] [CrossRef]
- Xu, Z.; Jia, H.; Yan, L.; Chu, Z.; Zhou, H. Synergistic effects of organically modified montmorillonite in combination with metal oxides on the fire safety enhancement of intumescent flame-retarded epoxy resins. J. Vinyl Addit. Technol. 2021, 27, 161–173. [Google Scholar] [CrossRef]
- Gu, L.; Yu, Q.; Zhang, L. Preparation and characterization of the halogen-free, smoke suppression, organic–inorganic hybrid flame-retardant expandable polystyrene materials. J. Appl. Polym. Sci. 2020, 137, 1–13. [Google Scholar] [CrossRef]
- Wang, H.; Wang, E.; Liu, Z.; Gao, D.; Yuan, R.; Sun, L.; Zhu, Y. A novel carbon nanotubes reinforced superhydrophobic and superoleophilic polyurethane sponge for selective oil-water separation through a chemical fabrication. J. Mater. Chem. A 2015, 3, 266–273. [Google Scholar] [CrossRef]
- Hamdani-Devarennes, S.; El Hage, R.; Dumazert, L.; Sonnier, R.; Ferry, L.; Lopez-Cuesta, J.M.; Bert, C. Water-based flame retardant coating using nano-boehmite for expanded polystyrene (EPS) foam. Prog. Org. Coat. 2016, 99, 32–46. [Google Scholar] [CrossRef]
- Bevas, C.J.; Abel, M.L.; Jacobs, I.; Watts, J.F. An interfacial chemistry study of methylene diphenyl diisocyanate and tantalum for heat exchanger applications. Surf. Interface Anal. 2020, 52, 685–693. [Google Scholar] [CrossRef]
- Frazier, C. Isocyanate Wood Binders. Handb. Adhes. Technol. Revis. Expand. 2003, 33, 1–14. [Google Scholar] [CrossRef]
- Tan, R. The use of p-MDI resin in MDF manufacture. Bachelor’s Thesis, University of British Columbia, Vancouver, BC, Canada, 12 April 2012. [Google Scholar] [CrossRef]
- USA Environmental Protection Agency. Methylene Diphenyl Diisocyanate (MDI) Action Plan; USA Environmental Protection Agency: Washington, DC, USA, 2011; pp. 1–15.
- Thomas, G. Thermal properties of gypsum plasterboard at high temperatures. Fire Mater. 2002, 26, 37–45. [Google Scholar] [CrossRef]
- Ji, W.; Yao, Y.; Guo, J.; Fei, B.; Gu, X.; Li, H.; Sun, J.; Zhang, S. Toward an understanding of how red phosphorus and expandable graphite enhance the fire resistance of expandable polystyrene foams. J. Appl. Polym. Sci. 2020, 137, 49045. [Google Scholar] [CrossRef]
- Feng, C.M.; Zhang, Y.; Lang, D.; Liu, S.W.; Chi, Z.G.; Xu, J.R. Flame retardant mechanism of a novel intumescent flame retardant polypropylene. Procedia Eng. 2013, 52, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Liu, Y.; Zhang, L.; Wang, H. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016, 51, 5857–5871. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, W.; Cheng, Y. Influence of magnesium hydroxide on thermal decomposition of intumescent fire-retardant epoxy coatings. J. Thermoplast. Compos. Mater. 2016, 29, 1151–1164. [Google Scholar] [CrossRef]
- Patrick Lim, W.K.; Mariatti, M.; Chow, W.S.; Mar, K.T. Effect of intumescent ammonium polyphosphate (APP) and melamine cyanurate (MC) on the properties of epoxy/glass fiber composites. Compos. Part B Eng. 2012, 43, 124–128. [Google Scholar] [CrossRef]
- Ying, Z.; Zygimantas, G.; Claus, E.W.; Kim, D.-J.; Louise, R.; Søren, K. Interaction between calcium carbonate and ammonium polyphosphate in low-borate concentration hydrocarbon intumescent coatings. Fire Mater 2021. (In Press) [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.; Gaan, S. Recent studies on the decomposition and strategies of smoke and toxicity suppression for polyurethane based materials. RSC Adv. 2016, 6, 74742–74756. [Google Scholar] [CrossRef] [Green Version]
- An, W.; Jiang, L.; Sun, J.; Liew, K.M. Correlation analysis of sample thickness, heat flux, and cone calorimetry test data of polystyrene foam. J. Therm. Anal. Calorim. 2015, 119, 229–238. [Google Scholar] [CrossRef]
- Yu, T.; Hu, C.; Chen, X.; Li, Y. Effect of diisocyanates as compatibilizer on the properties of ramie/poly(lactic acid) (PLA) composites. Compos. Part A Appl. Sci. Manuf. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Shao, Z.B.; Deng, C.; Tan, Y.; Yu, L.; Chen, M.J.; Chen, L.; Wang, Y.Z. Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J. Mater. Chem. A 2014, 2, 13955–13965. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, W.; Meng, W.; Xie, W.; Cui, Y.; Xu, J.; Qu, H. Core-shell graphitic carbon nitride/zinc phytate as a novel effcient flame retardant for fire safety and smoke suppression in epoxy resin. Polymers 2020, 12, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Wang, P.; Xia, L.; Jian, R.; Ai, Y.; Zheng, X.; Chen, G.; Wang, J. Flame-retarding epoxy resin with an efficient P/N/S-containing flame retardant: Preparation, thermal stability, and flame retardance. Polym. Degrad. Stab. 2018, 149, 69–77. [Google Scholar] [CrossRef]
- Zhao, H.B.; Liu, B.W.; Wang, X.L.; Chen, L.; Wang, X.L.; Wang, Y.Z. A flame-retardant-free and thermo-cross-linkable copolyester: Flame-retardant and anti-dripping mode of action. Polymer 2014, 55, 2394–2403. [Google Scholar] [CrossRef]
- Yuan, G.; Yang, B.; Chen, Y.; Jia, Y. Preparation of novel phosphorus-nitrogen-silicone grafted graphene oxide and its synergistic effect on intumescent flame-retardant polypropylene composites. RSC Adv. 2018, 8, 36286–36297. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Duan, H.; Chen, Y.; Ji, S.; Cao, J.; Ma, H. A P/N/S-containing high-efficiency flame retardant endowing epoxy resin with excellent flame retardance, mechanical properties and heat resistance. Compos. Part B Eng. 2020, 199, 108228. [Google Scholar] [CrossRef]
- Feng, C.; Liang, M.; Jiang, J.; Huang, J.; Liu, H. Flame retardant properties and mechanism of an efficient intumescent flame retardant PLA composites. Polym. Adv. Technol. 2016, 27, 693–700. [Google Scholar] [CrossRef]
- Ran, J.; Qiu, J.; Xie, H.; Lai, X.; Li, H.; Zeng, X. Combination effect of zirconium phosphate nanosheet and PU-coated carbon fiber on flame retardancy and thermal behavior of PA46/PPO alloy. Compos. Part B Eng. 2019, 166, 621–632. [Google Scholar] [CrossRef]
- Huang, J.; Tang, Q.; Liao, W.; Wang, G.; Wei, W.; Li, C. Green Preparation of Expandable Graphite and Its Application in Flame-Resistance Polymer Elastomer. Ind. Eng. Chem. Res. 2017, 56, 5253–5261. [Google Scholar] [CrossRef]
- Focke, W.W.; Badenhorst, H.; Mhike, W.; Kruger, H.J.; Lombaard, D. Characterization of commercial expandable graphite fire retardants. Thermochim. Acta 2014, 584, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Price, D.; Liu, Y.; Milnes, G.J.; Hull, R.; Kandola, B.K.; Horrocks, A.R. An investigation into the mechanism of flame retardancy and smoke suppression by melamine in flexible polyurethane foam. Fire Mater. 2002, 26, 201–206. [Google Scholar] [CrossRef]










| Sample Code | PER (g) | CaCO3 (g) | MC (g) |
|---|---|---|---|
| IFR1 | 7.5 | 7.5 | 0 |
| IFR2 | 0 | 7.5 | 7.5 |
| IFR3 | 5 | 5 | 5 |
| Sample Code | Initial Wt. of EPS (g) | Final Weight(g) after Drying (1:3 ratio) | Wt. of IFR Coated on EPS (g) | Density (kg/m3) |
|---|---|---|---|---|
| EPS | 13 | - | - | 26 |
| EPS/IFR3 | 13 | 35.5 | 22.5 | 71 |
| EPS/MDI/IFR3 | 14.5 | 41.90 | 27.4 | 83 |
| Sample Code | PHRR (kW/m2) | THR (MJ/m2) | FIGRA (W/m−2s) | TSP (m2) |
|---|---|---|---|---|
| EPS | 295.5 | 42.3 | 6274.0 | 10.99 |
| EPS/IRF1 | 70.2 | 17.6 | 3086.2 | 0.457 |
| EPS/IRF2 | 70.3 | 12.3 | 3176.7 | 0.196 |
| EPS/IRF3 | 65 | 10.9 | 2441.9 | 0.394 |
| EPS/MDI/IFR3 | 57.6 | 7.3 | 2027.0 | 0.133 |
| Sample Name | C% | O% | N% | P% | Ca% | S% | Mg% | Si% | C/O | P/C | N/C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EPS/IFR3 | 86.06 | 8.96 | 1.31 | 0.78 | 0.33 | 0.15 | 0.14 | 2.26 | 9.60 | 0.009 | 0.015 |
| EPS/MDI/IFR3 | 88.61 | 7.6 | 2.7 | 1.31 | 0.4 | 0.24 | 0.13 | 0.01 | 11.39 | 0.015 | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhoite, S.P.; Kim, J.; Jo, W.; Bhoite, P.H.; Mali, S.S.; Park, K.-H.; Hong, C.-K. Expanded Polystyrene Beads Coated with Intumescent Flame Retardant Material to Achieve Fire Safety Standards. Polymers 2021, 13, 2662. https://doi.org/10.3390/polym13162662
Bhoite SP, Kim J, Jo W, Bhoite PH, Mali SS, Park K-H, Hong C-K. Expanded Polystyrene Beads Coated with Intumescent Flame Retardant Material to Achieve Fire Safety Standards. Polymers. 2021; 13(16):2662. https://doi.org/10.3390/polym13162662
Chicago/Turabian StyleBhoite, Sangram P., Jonghyuck Kim, Wan Jo, Pravin H. Bhoite, Sawanta S. Mali, Kyu-Hwan Park, and Chang-Kook Hong. 2021. "Expanded Polystyrene Beads Coated with Intumescent Flame Retardant Material to Achieve Fire Safety Standards" Polymers 13, no. 16: 2662. https://doi.org/10.3390/polym13162662