Cigarette Butt Waste as Material for Phase Inverted Membrane Fabrication Used for Oil/Water Emulsion Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
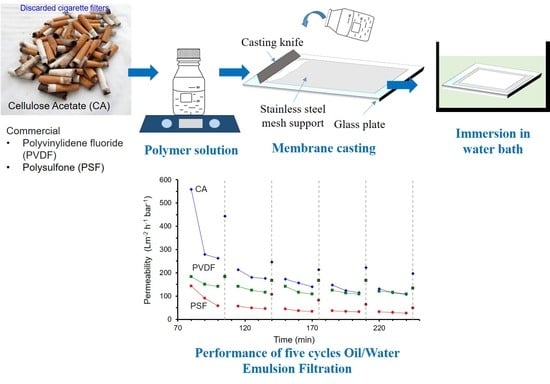
2.2. Membrane Preparation
2.3. Membrane Filtration Setup
2.4. Membrane Characterization
2.5. Membrane Fouling Identification
3. Results and Discussion
3.1. Surface and Cross-Section Morphologies
3.2. Membrane Pore Size and Distribution
3.3. Surface Contact Angle
3.4. Fourier Transform Infrared
3.5. Energy Dispersive X-ray Spectroscopy
3.6. Clean Water Permeability
3.7. Filtration Performance
3.8. Rejection Performance
3.9. Membrane Fouling Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torkashvand, J.; Farzadkia, M.; Sobhi, H.R.; Esrafili, A. Littered cigarette butt as a well-known hazardous waste: A comprehensive systematic review. J. Hazard. Mater. 2020, 383, 121242. [Google Scholar] [CrossRef]
- Torkashvand, J.; Godini, K.; Jafari, A.J.; Esrafili, A.; Farzadkia, M. Assessment of littered cigarette butt in urban environment, using of new cigarette butt pollution index (CBPI). Sci. Total Environ. 2021, 769, 144864. [Google Scholar] [CrossRef] [PubMed]
- Kurmus, H.; Mohajerani, A. The toxicity and valorization options of cigarette butts. Waste Manag. 2020, 104, 104–118. [Google Scholar] [CrossRef]
- Hamzah, Y.; Umar, L. Preparation of creating active carbon from cigarette filter waste using microwave-induced KOH activation. J. Phys. Conf. Ser. 2017, 853, 012027. [Google Scholar] [CrossRef] [Green Version]
- Marinello, S.; Lolli, F.; Gamberini, R.; Rimini, B. A second life for cigarette butts? A review of recycling solutions. J. Hazard. Mater. 2020, 384, 121245. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jia, C.; Zhu, X.; Zhang, S. Utilization of cigarette butt waste as functional carbon precursor for supercapacitors and adsorbents. J. Clean. Prod. 2020, 256, 120326. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef] [Green Version]
- Um, M.-J.; Yoon, S.-H.; Lee, C.-H.; Chung, K.-Y.; Kim, J.-J. Flux enhancement with gas injection in crossflow ultrafiltration of oily wastewater. Water Res. 2001, 35, 4095–4101. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Barambu, N.U.; Bilad, M.R.; Bustam, M.A.; Huda, N.; Jaafar, J.; Narkkun, T.; Faungnawakij, K. Development of Polysulfone Membrane via Vapor-Induced Phase Separation for Oil/Water Emulsion Filtration. Polymers 2020, 12, 2519. [Google Scholar] [CrossRef] [PubMed]
- Nawi, N.I.M.; Sait, N.R.; Bilad, M.R.; Shamsuddin, N.; Jaafar, J.; Nordin, N.A.H.; Narkkun, T.; Faungnawakij, K.; Mohshim, D.F. Polyvinylidene Fluoride Membrane Via Vapour Induced Phase Separation for Oil/Water Emulsion Filtration. Polymers 2021, 13, 427. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Ong Amat, S.; Bilad, M.R.; Nordin, N.A.H.M.; Shamsuddin, N.; Prayogi, S.; Narkkun, T.; Faungnawakij, K. Development of Polyvinylidene Fluoride Membrane via Assembly of Tannic Acid and Polyvinylpyrrolidone for Filtration of Oil/Water Emulsion. Polymers 2021, 13, 976. [Google Scholar] [CrossRef] [PubMed]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2011, 19, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Saljoughi, E.; Sadrzadeh, M.; Mohammadi, T. Effect of preparation variables on morphology and pure water permeation flux through asymmetric cellulose acetate membranes. J. Membr. Sci. 2009, 326, 627–634. [Google Scholar] [CrossRef]
- Mohajerani, A.; Kadir, A.A.; Larobina, L. A practical proposal for solving the world’s cigarette butt problem: Recycling in fired clay bricks. Waste Manag. 2016, 52, 228–244. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, I. Adopting a Circular Economy: Current Practices and Future Perspectives. Soc. Sci. 2019, 8, 328. [Google Scholar] [CrossRef] [Green Version]
- Cucciniello, R.; Cespi, D. Recycling within the Chemical Industry: The Circular Economy Era. Recycling 2018, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Cui, M.; Shen, Y.; Zhu, G.; Luo, L.; Li, M.; Li, J. Waste cigarette filter as nanofibrous membranes for on-demand immiscible oil/water mixtures and emulsions separation. J. Colloid Interface Sci. 2019, 549, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Abd Halim, N.S.; Wirzal, M.D.H.; Hizam, S.M.; Bilad, M.R.; Nordin, N.A.H.M.; Sambudi, N.S.; Putra, Z.A.; Yusoff, A.R.M. Recent Development on Electrospun Nanofiber Membrane for Produced Water Treatment: A review. J. Environ. Chem. Eng. 2021, 9, 104613. [Google Scholar] [CrossRef]
- Bilad, M.R.; Westbroek, P.; Vankelecom, I.F.J. Assessment and optimization of electrospun nanofiber-membranes in a membrane bioreactor (MBR). J. Membr. Sci. 2011, 380, 181–191. [Google Scholar] [CrossRef]
- Mohd Asri, M.A.N.; Abd Halim, N.S.; Wirzal, M.D.H.; Mohd Yusoff, A.R.; Bilad, M.R. Thermal Annealing Surface Modification: Effect on Surface and Performance of Electrospun Nylon 6,6 Nanofiber Membrane for Wastewater Treatment. J. Penelit. Dan Pengkaj. Ilmu Pendidik. E-St. 2021, 5, 56. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Judd, S.; Judd, C. Commercial Technologies. In The MBR Book; Elsevier: Amsterdam, The Netherlands, 2011; pp. 289–357. ISBN 978-0-08-096682-3. [Google Scholar]
- Pichot, R.; Spyropoulos, F.; Norton, I.T. O/W emulsions stabilised by both low molecular weight surfactants and colloidal particles: The effect of surfactant type and concentration. J. Colloid Interface Sci. 2010, 352, 128–135. [Google Scholar] [CrossRef]
- Mazinani, S.; Darvishmanesh, S.; Ehsanzadeh, A.; Van der Bruggen, B. Phase separation analysis of Extem/solvent/non-solvent systems and relation with membrane morphology. J. Membr. Sci. 2017, 526, 301–314. [Google Scholar] [CrossRef]
- Barzin, J.; Sadatnia, B. Theoretical phase diagram calculation and membrane morphology evaluation for water/solvent/polyethersulfone systems. Polymer 2007, 48, 1620–1631. [Google Scholar] [CrossRef]
- Chen, W.; Su, Y.; Zheng, L.; Wang, L.; Jiang, Z. The improved oil/water separation performance of cellulose acetate-graft-polyacrylonitrile membranes. J. Membr. Sci. 2009, 337, 98–105. [Google Scholar] [CrossRef]
- Li, F.; Gao, R.; Wu, T.; Li, Y. Role of layered materials in emulsified oil/water separation and anti-fouling performance of modified cellulose acetate membranes with hierarchical structure. J. Membr. Sci. 2017, 543, 163–171. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Supercritical Phase Inversion: A Powerful Tool for Generating Cellulose Acetate-AgNO3 Antimicrobial Membranes. Materials 2020, 13, 1560. [Google Scholar] [CrossRef] [Green Version]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Pornea, A.M.; Puguan, J.M.C.; Deonikar, V.G.; Kim, H. Robust Janus nanocomposite membrane with opposing surface wettability for selective oil-water separation. Sep. Purif. Technol. 2020, 236, 116297. [Google Scholar] [CrossRef]
- Elnabawy, E.; Elsherbiny, I.M.A.; Abdelsamad, A.M.A.; Anis, B.; Hassan, A.; Ulbricht, M.; Khalil, A.S.G. Tailored CNTs Buckypaper Membranes for the Removal of Humic Acid and Separation of Oil-In-Water Emulsions. Membranes 2020, 10, 97. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A Review on Membrane Technology and Chemical Surface Modification for the Oily Wastewater Treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, R.; Karimi-Sabet, J.; Shariaty-Niassar, M.; Koochaki, M. Preparation and Characterization of Polyvinylidene Fluoride/Graphene Superhydrophobic Fibrous Films. Polymers 2015, 7, 1444–1463. [Google Scholar] [CrossRef] [Green Version]
- Guezguez, I.; Mrabet, B.; Ferjani, E. XPS and contact angle characterization of surface modified cellulose acetate membranes by mixtures of PMHS/PDMS. Desalination 2013, 313, 208–211. [Google Scholar] [CrossRef]
- Han, B.; Zhang, D.; Shao, Z.; Kong, L.; Lv, S. Preparation and characterization of cellulose acetate/carboxymethyl cellulose acetate blend ultrafiltration membranes. Desalination 2013, 311, 80–89. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Devanadera, K.P.O.; Duena, A.N.R.; Luo, Z.-Y.; Chiao, Y.-H.; Millare, J.C.; Aquino, R.R.; Huang, S.-H.; Lee, K.-R. Modifying Cellulose Acetate Mixed-Matrix Membranes for Improved Oil–Water Separation: Comparison between Sodium and Organo-Montmorillonite as Particle Additives. Membranes 2021, 11, 80. [Google Scholar] [CrossRef]
- Andrade, M.C.; Pereira, J.C.; de Almeida, N.; Marques, P.; Faria, M.; Gonçalves, M.C. Improving hydraulic permeability, mechanical properties, and chemical functionality of cellulose acetate-based membranes by co-polymerization with tetraethyl orthosilicate and 3-(aminopropyl)triethoxysilane. Carbohydr. Polym. 2021, 261, 117813. [Google Scholar] [CrossRef]
- Hołda, A.K.; De Roeck, M.; Hendrix, K.; Vankelecom, I.F.J. The influence of polymer purity and molecular weight on the synthesis of integrally skinned polysulfone membranes. J. Membr. Sci. 2013, 446, 113–120. [Google Scholar] [CrossRef]
- Fernandes, C.S.; Md Nordin, N.A.H.; Bilad, M.R.; Matsuura, T.; Putra, Z.A.; Wirzal, M.D.H.; Jaafar, J. Explication of hydrophobic silica as effective pore former for membrane fabrication. Appl. Surf. Sci. Adv. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Ifelebuegu, A.; Lale, E.; Mbanaso, F.; Theophilus, S. Facile Fabrication of Recyclable, Superhydrophobic, and Oleophilic Sorbent from Waste Cigarette Filters for the Sequestration of Oil Pollutants from an Aqueous Environment. Processes 2018, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Nair, K.; Sambhudevan, S.; Shankar, B. Synthesis, Characterization and dye absorbing properties of cellulose acetate from used cigarette buds. Mater. Today Proc. 2019, 18, 5006–5011. [Google Scholar] [CrossRef]
- Nagasawa, H.; Omura, T.; Asai, T.; Kanezashi, M.; Tsuru, T. Filtration of surfactant-stabilized oil-in-water emulsions with porous ceramic membranes: Effects of membrane pore size and surface charge on fouling behavior. J. Membr. Sci. 2020, 610, 118210. [Google Scholar] [CrossRef]
- Mat Nawi, N.I.; Chean, H.M.; Shamsuddin, N.; Bilad, M.R.; Narkkun, T.; Faungnawakij, K.; Khan, A.L. Development of Hydrophilic PVDF Membrane Using Vapour Induced Phase Separation Method for Produced Water Treatment. Membranes 2020, 10, 121. [Google Scholar] [CrossRef]
- Cote, P.; Alam, Z.; Penny, J. Hollow fiber membrane life in membrane bioreactors (MBR). Desalination 2012, 288, 145–151. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P. Fabrication of cellulose acetate–zirconia hybrid membranes for ultrafiltration applications: Performance, structure and fouling analysis. Sep. Purif. Technol. 2010, 74, 230–235. [Google Scholar] [CrossRef]
- Margarito, M.T.; Beltran, A.B.; Huelgas-Orbecido, A. Characteristics and Performance of PTU-Cu Composite Membrane Fabricated through Simultaneous Complexation and Non-Solvent Induced Phase Separation. Polymers 2021, 13, 1743. [Google Scholar] [CrossRef] [PubMed]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]










| Membrane | Polymer | Solvent | Additives | Support |
|---|---|---|---|---|
| CA | 10 wt% of CA | 90 wt% of DMF | - | Stainless steel mesh |
| PSF | 18 wt% of PSF | 80.9 wt% of DMAc | 1 wt% of PEG and 0.1 wt% of LiCl | Non-woven support |
| PVDF | 15 wt% of PVDF | 85 wt% of DMAc | - | Non-woven support |
| Membrane | Composition (%) | |||
|---|---|---|---|---|
| C | F | O | S | |
| CA | 51.60 | 0.00 | 48.20 | 0.00 |
| PSF | 69.02 | 0.00 | 26.05 | 4.92 |
| PVDF | 55.55 | 42.84 | 1.61 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doyan, A.; Leong, C.L.; Bilad, M.R.; Kurnia, K.A.; Susilawati, S.; Prayogi, S.; Narkkun, T.; Faungnawakij, K. Cigarette Butt Waste as Material for Phase Inverted Membrane Fabrication Used for Oil/Water Emulsion Separation. Polymers 2021, 13, 1907. https://doi.org/10.3390/polym13121907
Doyan A, Leong CL, Bilad MR, Kurnia KA, Susilawati S, Prayogi S, Narkkun T, Faungnawakij K. Cigarette Butt Waste as Material for Phase Inverted Membrane Fabrication Used for Oil/Water Emulsion Separation. Polymers. 2021; 13(12):1907. https://doi.org/10.3390/polym13121907
Chicago/Turabian StyleDoyan, Aris, Chew Lee Leong, Muhammad Roil Bilad, Kiki Adi Kurnia, Susilawati Susilawati, Saiful Prayogi, Thanitporn Narkkun, and Kajornsak Faungnawakij. 2021. "Cigarette Butt Waste as Material for Phase Inverted Membrane Fabrication Used for Oil/Water Emulsion Separation" Polymers 13, no. 12: 1907. https://doi.org/10.3390/polym13121907