Influence of the Impregnation Technique on the Release of Esomeprazole from Various Bioaerogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Alginate, Pectin, and Chitosan Gels
2.2.2. Preparation of Alginate and Pectin Gels Coated with Chitosan
2.2.3. Supercritical Drying of Gels
2.2.4. Impregnation of Esomeprazole
2.2.5. In Vitro Dissolution Tests
2.2.6. Characterization Methods
3. Results
3.1. Characterization of Materials
3.1.1. N2 Adsorption-Desorption Analysis
3.1.2. Thermal Analysis (Thermogravimetry and Differential Scanning Calorimetry Analysis)
3.1.3. Fourier Transform Infrared Spectroscopy
3.2. Esomeprazole Loadings
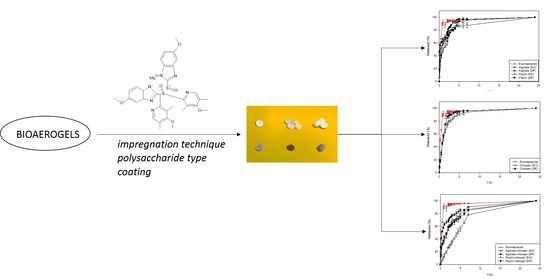
3.3. In Vitro Dissolution Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ülker, Z.; Erkey, C. An Emerging Platform for Drug Delivery: Aerogel Based Systems. J. Control. Release Off. J. Control. Release Soc. 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.A.; Rathbone, M.J. Overview of Controlled Release Mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Advances in Delivery Science and Technology; Springer US: Boston, MA, USA, 2012; pp. 19–43. ISBN 978-1-4614-0881-9. [Google Scholar]
- Duarte, A.R.C.; Costa, M.S.; Simplício, A.L.; Cardoso, M.M.; Duarte, C.M.M. Preparation of controlled release microspheres using supercritical fluid technology for delivery of anti-inflammatory drugs. Int. J. Pharm. 2006, 308, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical CO2 adsorption of non-steroidal anti-inflammatory drugs into biopolymer aerogels. J. CO2 Util. 2020, 36, 40–53. [Google Scholar] [CrossRef]
- Mehling, T.; Smirnova, I.; Guenther, U.; Neubert, R.H.H. Polysaccharide-based aerogels as drug carriers. J. Non-Cryst. Solids 2009, 50–51, 2472–2479. [Google Scholar] [CrossRef]
- Mudgil, D. Chapter 3—The Interaction Between Insoluble and Soluble Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Samaan, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 35–59. ISBN 978-0-12-805130-6. [Google Scholar]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- BeMiller, J.N. 14—Algins/Alginates. In Carbohydrate Chemistry for Food Scientists (Third Edition); BeMiller, J.N., Ed.; AACC International Press: Washington, DC, USA, 2019; pp. 293–301. ISBN 978-0-12-812069-9. [Google Scholar]
- Pantić, M.; Horvat, G.; Knez, Ž.; Novak, Z. Preparation and Characterization of Chitosan-Coated Pectin Aerogels: Curcumin Case Study. Molecules 2020, 25, 1187. [Google Scholar] [CrossRef] [Green Version]
- Alnaief, M.; Antonyuk, S.; Hentzschel, C.M.; Leopold, C.S.; Heinrich, S.; Smirnova, I. A novel process for coating of silica aerogel microspheres for controlled drug release applications. Microporous Mesoporous Mater. 2012, 160, 167–173. [Google Scholar] [CrossRef]
- Jennings, J.A.; Wells, C.M.; McGraw, G.S.; Velasquez Pulgarin, D.A.; Whitaker, M.D.; Pruitt, R.L.; Bumgardner, J.D. Chitosan coatings to control release and target tissues for therapeutic delivery. Ther. Deliv. 2015, 6, 855–871. [Google Scholar] [CrossRef]
- Sharif, S.N.M.; Hashim, N.; Isa, I.M.; Bakar, S.A.; Saidin, M.I.; Ahmad, M.S.; Mamat, M.; Hussein, M.Z. The influence of chitosan coating on the controlled release behaviour of zinc/aluminium-layered double hydroxide-quinclorac composite. Mater. Chem. Phys. 2020, 251, 123076. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Zairy, E.M.R. Chitosan as a Biomaterial—Structure, Properties, and Electrospun Nanofibers. Concepts Compd. Altern. Antibact. 2015. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P.; Hunt, A.; Macquarrie, D.; Clark, J. Chitosan Aerogels Exhibiting High Surface Area for Biomedical Application: Preparation, Characterization, and Antibacterial Study. Int. J. Polym. Mater. 2011, 60, 988–999. [Google Scholar]
- McKeage, K.; Blick, S.K.A.; Croxtall, J.D.; Lyseng-Williamson, K.A.; Keating, G.M. Esomeprazole. Drugs 2008, 68, 1571–1607. [Google Scholar] [CrossRef]
- Johnson, D.A. Review of esomeprazole in the treatment of acid disorders. Expert Opin. Pharmacother. 2003, 4, 253–264. [Google Scholar] [CrossRef]
- PubChem Esomeprazole. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9568614 (accessed on 3 November 2020).
- Li, J.; Zhao, J.; Hamer-Maansson, J.E.; Andersson, T.; Fulmer, R.; Illueca, M.; Lundborg, P. Pharmacokinetic properties of esomeprazole in adolescent patients aged 12 to 17 years with symptoms of gastroesophageal reflux disease: A randomized, open-label study. Clin. Ther. 2006, 28, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Esomeprazole Magnesium Trihydrate|DrugBank. Available online: https://go.drugbank.com/salts/DBSALT001836 (accessed on 3 November 2020).
- Jain, A.; Teja, M.N.R.; Pariyani, L.; Balamuralidhara, V.; Gupta, N.V. Formulation and Evaluation of Spray-Dried Esomeprazole Magnesium Microspheres. Trop. J. Pharm. Res. 2013, 12, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Ganure, A.L.; Subudhi, B.B.; Shukla, S. Preparation and characterization of pH-sensitive methyl methacrylate-g-starch/hydroxypropylated starch hydrogels: In vitro and in vivo study on release of esomeprazole magnesium. Drug Deliv. Transl. Res. 2015, 5, 243–256. [Google Scholar] [CrossRef]
- Xie, P.; Xie, Y.; Song, H.; Song, X.; Zuo, Y.; Qiu, Y.; Tang, X. Preparation and dissolution in vitro of esomeprazole zinc solid dispersion. Sichuan Da Xue Xue Bao Yi Xue Ban 2008, 39, 648–650. [Google Scholar]
- Tkalec, G.; Knez, Ž.; Novak, Z. Formation of polysaccharide aerogels in ethanol. RSC Adv. 2015, 5, 77362–77371. [Google Scholar] [CrossRef]
- Ega, J.K.; Vadde, R. Formulation and evaluation of esomeprazole magnesium delayed release tablets 40 mg. Int. J. Res. Appl. 2015, 2, 242–252. [Google Scholar]
- Gul, W.; Sajid, S.; Hamid, F.; Bhatti, S. Effect of acidic Ph. and heat on the degradation of omeprazole and Esomeprazole. Pharma Innov. J. 2015, 4, 19–21. [Google Scholar]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Encapsulation and drug release of poorly water soluble nifedipine from bio-carriers. J. Non-Cryst. Solids 2018, 481, 486–493. [Google Scholar] [CrossRef]
- Product Information: Esomeprazole Magnesium. Available online: https://www.webmd.com/drugs/2/drug-20537-4143/esomeprazole-magnesium-oral/esomeprazole-delayed-release-capsule-oral/details (accessed on 1 June 2021).
- Pantić, M.; Knez, Ž.; Novak, Z. Supercritical impregnation as a feasible technique for entrapment of fat-soluble vitamins into alginate aerogels. J. Non-Cryst. Solids 2016, 432, 519–526. [Google Scholar] [CrossRef]
- Sodeifian, G.; Detakhsheshpour, R.; Sajadian, S.A. Experimental study and thermodynamic modeling of Esomeprazole (proton-pump inhibitor drug for stomach acid reduction) solubility in supercritical carbon dioxide. J. Supercrit. Fluids 2019, 154, 104606. [Google Scholar] [CrossRef]
- U.S. Pharmacopeial Convention. Available online: http://www.usp.org/ (accessed on 21 July 2016).
- Gonçalves, V.S.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.M.; Smirnova, I. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. Fast production of high-methoxyl pectin aerogels for enhancing the bioavailability of low-soluble drugs. J. Supercrit. Fluids 2015, 106, 16–22. [Google Scholar] [CrossRef]
- García-González, C.A.; Carenza, E.; Zeng, M.; Smirnova, I.; Roig, A. Design of biocompatible magnetic pectin aerogel monoliths and microspheres. RSC Adv. 2012, 2, 9816–9823. [Google Scholar] [CrossRef]
- Groult, S.; Budtova, T. Tuning structure and properties of pectin aerogels. Eur. Polym. J. 2018, 108, 250–261. [Google Scholar] [CrossRef]
- Díez-Municio, M.; Montilla, A.; Herrero, M.; Olano, A.; Ibáñez, E. Supercritical CO2 impregnation of lactulose on chitosan: A comparison between scaffolds and microspheres form. J. Supercrit. Fluids 2011, 57, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Skieneh, J.; Khalili Najafabadi, B.; Horne, S.; Rohani, S. Crystallization of Esomeprazole Magnesium Water/Butanol Solvate. Molecules 2016, 21, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nguyen, H.; Baek, N.; Lee, B.-J. Enhanced gastric stability of esomeprazole by molecular interaction and modulation of microenvironmental pH with alkalizers in solid dispersion. Int. J. Pharm. 2017, 523, 189–202. [Google Scholar] [CrossRef] [PubMed]
- PantiĿ, M.; Kotnik, P.; Knez, Ž.; Novak, Z. High pressure impregnation of vitamin D3 into polysaccharide aerogels using moderate and low temperatures. J. Supercrit. Fluids 2016, 118, 171–177. [Google Scholar] [CrossRef]








| Sample | SBET, m2/g |
|---|---|
| Pectin | 501 ± 6 |
| Alginate | 220 ± 10 |
| Chitosan | 431 ± 11 |
| Pectin + chitosan | 314 ± 8 |
| Alginate + chitosan | 248 ± 12 |
| Bioaerogel | Loading | |
|---|---|---|
| SCI | DIF | |
| Pectin | 16.5 ± 1.0 | 19.5 ± 2.0 |
| Alginate | 10 ± 0.5 | 11.5 ± 0.5 |
| Chitosan | 15.5 ± 1.5 | 22 ± 1.5 |
| Pectin + chitosan | 2.5 ± 0.5 | 4 ± 0.5 |
| Alginate + chitosan | 9 ± 0.5 | 8.5 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantić, M.; Kravanja, K.A.; Knez, Ž.; Novak, Z. Influence of the Impregnation Technique on the Release of Esomeprazole from Various Bioaerogels. Polymers 2021, 13, 1882. https://doi.org/10.3390/polym13111882
Pantić M, Kravanja KA, Knez Ž, Novak Z. Influence of the Impregnation Technique on the Release of Esomeprazole from Various Bioaerogels. Polymers. 2021; 13(11):1882. https://doi.org/10.3390/polym13111882
Chicago/Turabian StylePantić, Milica, Katja Andrina Kravanja, Željko Knez, and Zoran Novak. 2021. "Influence of the Impregnation Technique on the Release of Esomeprazole from Various Bioaerogels" Polymers 13, no. 11: 1882. https://doi.org/10.3390/polym13111882